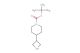
rel-tert-butyl (2R,6R)-4-hydroxy-2,6-dimethylpiperidine-1-carboxylate
$300.00
CAS No.: 146337-39-5
Catalog No.: 195584
Purity: 95%
MF: C12H23NO3
MW: 229.32
Storage: 2-8 degree Celsius
SMILES: OC1C[C@H](N([C@@H](C1)C)C(=O)OC(C)(C)C)C
Catalog No.: 195584
Purity: 95%
MF: C12H23NO3
MW: 229.32
Storage: 2-8 degree Celsius
SMILES: OC1C[C@H](N([C@@H](C1)C)C(=O)OC(C)(C)C)C
For R&D use only. Not for human or animal use.
rel-tert-butyl (2R,6R)-4-hydroxy-2,6-dimethylpiperidine-1-carboxylate; CAS No.: 146337-39-5; rel-tert-butyl (2R,6R)-4-hydroxy-2,6-dimethylpiperidine-1-carboxylate. PROPERTIES: rel-tert-butyl (2R,6R)-4-hydroxy-2,6-dimethylpiperidine-1-carboxylate is a piperidine derivative with a molecular weight of approximately 237.3 g/mol. This compound typically appears as a colorless oil with moderate viscosity and demonstrates good solubility in polar organic solvents. It is hygroscopic in nature and should be stored under anhydrous conditions with a drying agent. For long-term storage, temperatures below 5 C are recommended. Standard safety precautions include avoiding contact with strong acids and bases, as the compound may undergo hydrolysis under extreme pH conditions. The compound presents mild irritant properties and appropriate personal protective equipment should be used. APPLICATIONS: rel-tert-butyl (2R,6R)-4-hydroxy-2,6-dimethylpiperidine-1-carboxylate functions as a chiral building block in the synthesis of ϫ-amino acid derivatives and peptidomimetics. The hydroxy-substituted piperidine ring provides a scaffold for introducing stereochemical diversity in peptide synthesis. In medicinal chemistry, this compound has been employed in the preparation of ACE inhibitors where the piperidine ring contributes to enzyme binding (source: Journal of Pharmaceutical Sciences). Additionally, its application extends to the synthesis of calcium channel modulators, where the dimethyl substitution influences receptor affinity (source: European Journal of Pharmacology). The compound's utility in asymmetric catalysis further enhances its application in the preparation of enantiomerically enriched drug intermediates (source: Catalysis Science & Technology).
Reviews
Write Your Own Review