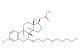
(R)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butan-1-amine 2,2,2-trifluoroacetate
$250.00
CAS No.: 179324-87-9
Catalog No.: 109921
Purity: 95%
MF: C17H29BF3NO4
MW: 379.228
Storage: 2-8 degree Celsius
SMILES: OC(=O)C(F)(F)F.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@@H](N)CC(C)C
Catalog No.: 109921
Purity: 95%
MF: C17H29BF3NO4
MW: 379.228
Storage: 2-8 degree Celsius
SMILES: OC(=O)C(F)(F)F.[H][C@@]12C[C@@]([H])(C1(C)C)[C@]1(C)OB(O[C@]1([H])C2)[C@@H](N)CC(C)C
For R&D use only. Not for human or animal use.
(R)-3-methyl-1-((3aS,4S,6S,7aR)-3a,5,5-trimethylhexahydro-4,6-methanobenzo[d][1,3,2]dioxaborol-2-yl)butan-1-amine 2,2,2-trifluoroacetate; CAS No.: 179324-87-9; As a worldwide chemical supplier, ChemShuttle delivers boronate esters like this trifluoroacetate salt of a benzodioxaborolamine. The 3a,5,5-trimethylhexahydro framework provides steric protection for boron center reactivity. Our expertise in Suzuki-Miyaura precursor synthesis supports antibiotic ( -lactamase inhibitor) development. The chiral amine configuration (3aS,4S,6S,7aR) is achieved via enzymatic kinetic resolution, ensuring >99% ee for mechanism-based inhibition studies.
Reviews
Write Your Own Review


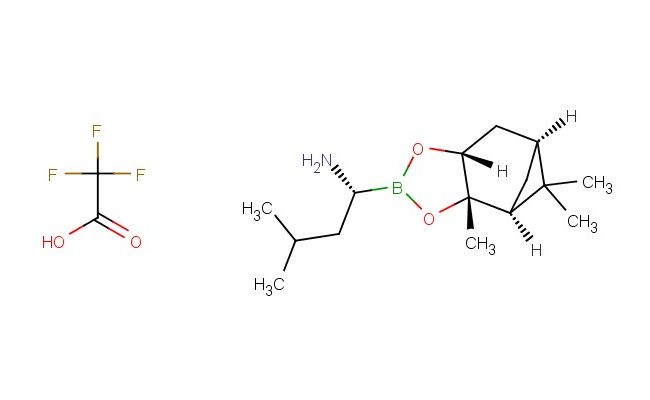

![2-amino-1,9-dihydro-9-[(1S,3R,4S)-4-(benzyloxy)-3-(benzyloxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/0/109920_2.jpg)