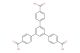
(1S,2S,3S,5S)-5-(2-amino-6-(benzyloxy)-9H-purin-9-yl)-3-(benzyloxy)-2-(benzyloxymethyl)cyclopentanol
$200.00
CAS No.: 142217-77-4
Catalog No.: 109916
Purity: 95%
MF: C32H33N5O4
MW: 551.647
Storage: 2-8 degree Celsius
SMILES: NC1=NC(OCC2=CC=CC=C2)=C2N=CN([C@H]3C[C@H](OCC4=CC=CC=C4)[C@@H](COCC4=CC=CC=C4)[C@@H]3O)C2=N1
Catalog No.: 109916
Purity: 95%
MF: C32H33N5O4
MW: 551.647
Storage: 2-8 degree Celsius
SMILES: NC1=NC(OCC2=CC=CC=C2)=C2N=CN([C@H]3C[C@H](OCC4=CC=CC=C4)[C@@H](COCC4=CC=CC=C4)[C@@H]3O)C2=N1
For R&D use only. Not for human or animal use.
(1S,2S,3S,5S)-5-(2-amino-6-(benzyloxy)-9H-purin-9-yl)-3-(benzyloxy)-2-(benzyloxymethyl)cyclopentanol; CAS No.: 142217-77-4; Specializing in pharma building blocks, ChemShuttle supplies this multi-benzyl protected cyclopentanol adenine derivative, a key intermediate for carbocyclic nucleoside synthesis. The stereochemical complexity (1S,2S,3S,5S) showcases our expertise in Johnson-Claisen rearrangements for cyclopentane ring formation. Our orthogonal protection strategy (three benzyl groups) allows sequential deprotection for selective functionalization, supporting antiviral and anticancer nucleotide analog development.
Reviews
Write Your Own Review



![(2R,3S,5S)-3-(benzyloxy)-5-[2-[[(4-methoxyphenyl)diphenylmethyl]amino]-6-(phenylmethoxy)-9H-purin-9-yl]-2-(benzyloxymethyl)cyclopentanol](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/0/109917_2.jpg)