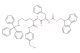
Fmoc-Phe-Lys(Trt)-PAB-PNP
$600.00
CAS No.: 1116086-09-9
Catalog No.: 198467
Purity: 95%
MF: C63H57N5O9
MW: 1028.175
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C1=CC=C(OC(=O)OCC2=CC=C(C=C2)NC([C@H](CCCCNC(C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)NC([C@H](CC2=CC=CC=C2)NC(OCC2C3=CC=CC=C3C=3C=CC=CC23)=O)=O)=O)C=C1
Catalog No.: 198467
Purity: 95%
MF: C63H57N5O9
MW: 1028.175
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C1=CC=C(OC(=O)OCC2=CC=C(C=C2)NC([C@H](CCCCNC(C2=CC=CC=C2)(C2=CC=CC=C2)C2=CC=CC=C2)NC([C@H](CC2=CC=CC=C2)NC(OCC2C3=CC=CC=C3C=3C=CC=CC23)=O)=O)=O)C=C1
CAS No.: 1116086-09-9; Fmoc-Phe-Lys(Trt)-PAB-PNP. PROPERTIES: Fmoc-Phe-Lys(Trt)-PAB-PNP is a protected peptide derivative with molecular formula C50H49N5O7 and molecular weight 847.93 g/mol. It typically appears as a white to off-white powder with limited solubility in water, requiring dimethylformamide or dimethyl sulfoxide for dissolution. The compound is sensitive to base and should be stored at 2-8 C in a tightly sealed container under inert atmosphere. Safety precautions include avoiding skin and eye contact due to potential irritancy and using protective equipment during handling. APPLICATIONS: In peptide chemistry, this compound serves as a building block for solid-phase peptide synthesis. The Fmoc group allows for temporary protection of the amino terminus, while the Trt group protects the epsilon-amino group of lysine. The PAB (para-aminobenzyl) group and PNP (para-nitrophenyl) ester facilitate conjugation and deprotection steps. In pharmaceutical research, it is used to synthesize complex peptide conjugates where controlled protection and deprotection strategies are essential. The compound enables synthesis of peptides with site-specific modifications for drug delivery applications, such as antibody-peptide conjugates or cell-penetrating peptide fusions. Additionally, in bioconjugation, the PAB group provides a handle for attaching to other molecules via its amine functionality after deprotection. The Fmoc and Trt protection strategy allows stepwise assembly of peptides with minimal side reactions, preserving peptide integrity during synthesis. In materials science, the compound can be incorporated into peptide-based materials where controlled deprotection triggers structural changes or releases bioactive molecules. (Peptide synthesis literature and bioconjugation chemistry articles)
Reviews
Write Your Own Review