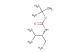
(R)-tert-butyl (1-aminopropan-2-yl)carbamate
$200.00
CAS No.: 100927-10-4
Catalog No.: TQP2152
Purity: 95%
MF: C8H18N2O2
MW: 174.244
Storage: 2-8 degree Celsius
SMILES: NC[C@@H](C)NC(OC(C)(C)C)=O
Catalog No.: TQP2152
Purity: 95%
MF: C8H18N2O2
MW: 174.244
Storage: 2-8 degree Celsius
SMILES: NC[C@@H](C)NC(OC(C)(C)C)=O
For R&D use only. Not for human or animal use.
CAS NO.: 100927-10-4; (R)-tert-butyl (1-aminopropan-2-yl)carbamate. PROPERTIES: (R)-tert-butyl (1-aminopropan-2-yl)carbamate appears as a white crystalline powder with a characteristic amine odor. This chiral compound exhibits low solubility in water but dissolves readily in organic solvents such as dichloromethane and ethyl acetate. Its molecular formula is C7H15N2O2, and it has a molecular weight of 163.20 g/mol. The compound is hygroscopic and should be stored at 2-8 degree Celsius in a tightly sealed container to prevent moisture absorption. When handling this substance, protective equipment including gloves, eye protection, and lab coats are recommended due to its potential to cause respiratory and skin irritation upon prolonged exposure. The compound is stable under normal laboratory conditions but decomposes upon exposure to strong acids or bases. APPLICATIONS: (R)-tert-butyl (1-aminopropan-2-yl)carbamate serves as a valuable intermediate in asymmetric synthesis, particularly in the preparation of enantiomerically pure amines. Its chiral structure makes it ideal for applications in pharmaceutical development where stereochemistry is critical. The compound is frequently used in the synthesis of (R)-configured amino alcohols, which are key building blocks for various drugs including beta-blockers and antiviral agents. In combinatorial chemistry, (R)-tert-butyl (1-aminopropan-2-yl)carbamate facilitates the creation of diverse compound libraries through its ability to undergo selective reactions while maintaining stereochemical integrity. Additionally, its Boc protection group allows for temporary protection of the amine functionality during multi-step syntheses, enabling sequential transformations without racemization. Researchers in medicinal chemistry utilize this compound to synthesize bioactive molecules with specific spatial configurations, enhancing both efficacy and safety profiles of resulting pharmaceuticals.
Reviews
Write Your Own Review