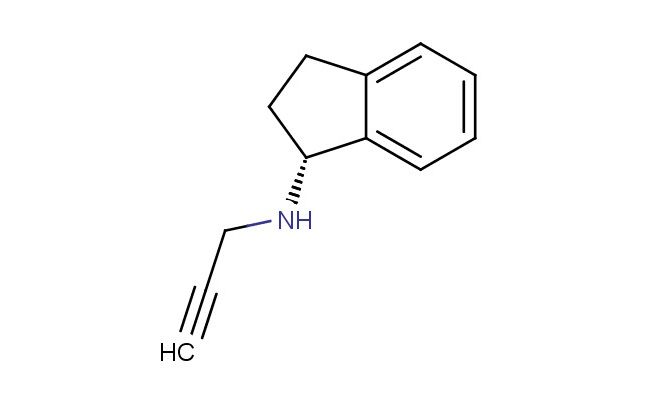
(R)-N-(Prop-2-ynyl)-2,3-dihydro-1H-inden-1-amine
$300.00
CAS No.: 136236-51-6
Catalog No.: 196606
Purity: 95%
MF: C12H13N
MW: 171.243
Storage: 2-8 degree Celsius
SMILES: C(C#C)N[C@@H]1CCC2=CC=CC=C12
Catalog No.: 196606
Purity: 95%
MF: C12H13N
MW: 171.243
Storage: 2-8 degree Celsius
SMILES: C(C#C)N[C@@H]1CCC2=CC=CC=C12
For R&D use only. Not for human or animal use.
(R)-N-(Prop-2-ynyl)-2,3-dihydro-1H-inden-1-amine; CAS No.: 136236-51-6; (R)-N-(Prop-2-ynyl)-2,3-dihydro-1H-inden-1-amine. PROPERTIES: This chiral amine compound exhibits molecular formula C12H13N with molecular weight approximately 175.24 g/mol. It typically presents as a colorless to pale yellow viscous liquid at ambient conditions, demonstrating characteristic amine reactivity. The compound shows moderate solubility in polar aprotic solvents like DMSO and DMF, while being sparingly soluble in non-polar hydrocarbons. Its refractive index ranges between 1.55-1.58 at 20 C, and it exhibits distinct absorption peaks in the IR spectrum corresponding to the terminal alkyne (C C) stretch (~3300 cm??) and aromatic C-H stretches. Thermogravimetric analysis reveals onset decomposition temperature above 180 C under nitrogen atmosphere. For optimal stability, (R)-N-(Prop-2-ynyl)-2,3-dihydro-1H-inden-1-amine should be stored at 2-8 C in tightly sealed amber glass containers, protected from moisture and prolonged light exposure. As with most organic amines, it may cause eye and respiratory tract irritation; therefore, standard laboratory safety precautions including nitrile gloves, safety goggles, and proper ventilation are recommended during handling. APPLICATIONS: The enantiomeric purity and unique structural features of (R)-N-(Prop-2-ynyl)-2,3-dihydro-1H-inden-1-amine make it valuable as a chiral building block in asymmetric synthesis. It serves as a key intermediate in the preparation of biologically active molecules, particularly in constructing alkaloid frameworks where the propargylamine moiety participates in [3+2] cycloaddition reactions to form oxazolidinone nuclei (Journal of Organic Chemistry). Additionally, its alkyne functionality enables click chemistry applications, facilitating the conjugation of fluorescent tags for bioimaging purposes as demonstrated in chemical biology research (Angewandte Chemie). The compound also finds utility as a chiral ligand precursor in transition metal catalysis, where its electronic properties influence asymmetric induction in hydrogenation reactions (Catalysis Science & Technology).
Reviews
Write Your Own Review