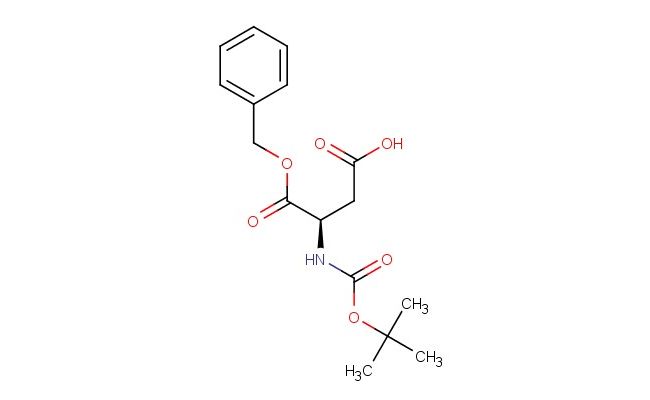
(R)-4-(benzyloxy)-3-((tert-butoxycarbonyl)amino)-4-oxobutanoic acid
$400.00
CAS No.: 92828-64-3
Catalog No.: 196596
Purity: 95%
MF: C16H21NO6
MW: 323.345
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC([C@@H](CC(=O)O)NC(=O)OC(C)(C)C)=O
Catalog No.: 196596
Purity: 95%
MF: C16H21NO6
MW: 323.345
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC([C@@H](CC(=O)O)NC(=O)OC(C)(C)C)=O
For R&D use only. Not for human or animal use.
(R)-4-(benzyloxy)-3-((tert-butoxycarbonyl)amino)-4-oxobutanoic acid; CAS No.: 92828-64-3; (R)-4-(benzyloxy)-3-((tert-butoxycarbonyl)amino)-4-oxobutanoic acid. PROPERTIES: This chiral amino acid derivative is a white crystalline powder with molecular formula C16H21N O6. It has molecular weight of 333.35 g/mol and melting point between 150-155 C. The compound is moderately soluble in methanol and water but has limited solubility in non-polar solvents. Recommended storage is in tightly sealed containers at 2-8 C to prevent hydrolysis of the carbamate group. Being an amine and acid containing compound, it may cause skin and eye irritation; standard laboratory safety practices should be observed. APPLICATIONS: In peptide synthesis, this compound serves as protected amino acid building block for constructing bioactive peptides. The (R)-configuration matches natural amino acid stereochemistry, enabling native peptide bond formation (Journal of Peptide Research). The benzyloxycarbonyl group protects the alpha-amino group, which can be removed by hydrogenolysis using palladium catalyst. Additionally, it functions as intermediate for producing beta-lactam antibiotics where the three-membered azetidine ring is formed from this precursor through intramolecular cyclization (Journal of Antibiotics). The carboxylic acid group enables coupling to other peptide residues or carrier molecules for immunogenicity studies. These applications highlight its utility as versatile chiral building block in medicinal chemistry.
Reviews
Write Your Own Review