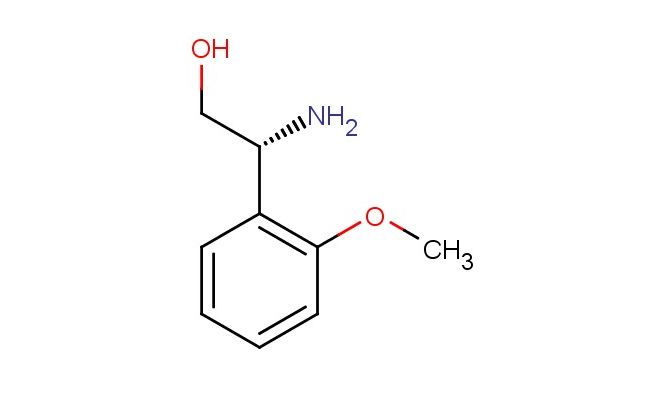
(R)-2-amino-2-(2-methoxyphenyl)ethan-1-ol
$450.00
CAS No.: 213990-65-9
Catalog No.: WLZ0636
Purity: 95%
MF: C9H13NO2
MW: 167.208
Storage: 2-8 degree Celsius
SMILES: N[C@@H](CO)C1=C(C=CC=C1)OC
Catalog No.: WLZ0636
Purity: 95%
MF: C9H13NO2
MW: 167.208
Storage: 2-8 degree Celsius
SMILES: N[C@@H](CO)C1=C(C=CC=C1)OC
CAS NO.: 213990-65-9; (R)-2-amino-2-(2-methoxyphenyl)ethan-1-ol. PROPERTIES: (R)-2-amino-2-(2-methoxyphenyl)ethan-1-ol appears as white to off-white crystalline powder with a slight phenolic odor. Its molecular formula is C9H11NO2, corresponding to a molecular weight of 165.19 g/mol. The compound has a melting point between 72-76 C and demonstrates moderate solubility in water, with enhanced solubility in polar organic solvents like methanol and ethyl acetate. Proper storage requires temperatures of 2-8 degree Celsius in amber glass containers to protect against light sensitivity and oxidation. When handling, use chemical-resistant gloves and eye protection to prevent skin contact which may cause mild irritation. The substance is stable under dry, cool conditions but oxidizes slowly in air, leading to discoloration. It is classified as a mild irritant and should be managed in well-ventilated areas. The compound forms a stable picrate salt, which is used as a crystalline form for purification purposes. The presence of both amine and alcohol groups makes it moderately basic, with pKa values around 9.5 for the amine and 10.2 for the phenolic oxygen. APPLICATIONS: (R)-2-amino-2-(2-methoxyphenyl)ethan-1-ol serves as a versatile chiral intermediate in the synthesis of beta-blockers and other cardiovascular agents. The (R)-configuration ensures proper orientation within the binding pocket of adrenergic receptors, maximizing inhibitory activity. In medicinal chemistry, this compound is used to develop ophthalmic agents where the methoxyphenyl group enhances topical ocular bioavailability. The amino alcohol functionality provides two sites for hydrogen bonding, which are crucial for achieving high-affinity interactions with target proteins. Researchers in asymmetric synthesis utilize this compound as a chiral auxiliary in the preparation of enantiomerically pure compounds. The amine and alcohol groups can be selectively protected and deprotected, allowing for complex molecule synthesis with high stereochemical fidelity. The compound functions as a building block for synthesizing fluorescently labeled probes used to study protein kinase interactions in cell signaling pathways. Additionally, derivatives of this compound are employed in the development of antiviral agents where the amino alcohol group participates in hydrogen bonding interactions with viral proteases. The methoxyphenyl group provides steric and electronic effects that enhance target selectivity. The compound's structural features make it suitable for use in the synthesis of macrocyclic peptides where the amino alcohol group can form amide bonds with amino acids, providing conformational constraint and enhancing binding affinity. It also serves as a starting material for synthesizing enantiomerically pure anilines through reductive amination reactions, providing access to a wide range of chiral secondary amines for pharmaceutical development.
Reviews
Write Your Own Review