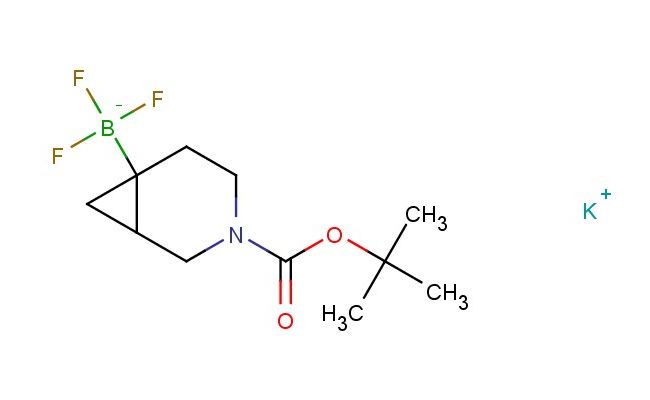
potassium (3-(tert-butoxycarbonyl)-3-azabicyclo[4.1.0]heptan-6-yl)trifluoroborate
$300.00
CAS No.: 2095504-46-2
Catalog No.: 195818
Purity: 95%
MF: C11H18BF3KNO2
MW: 303.174
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1CC2CC2(CC1)[B-](F)(F)F.[K+]
Catalog No.: 195818
Purity: 95%
MF: C11H18BF3KNO2
MW: 303.174
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1CC2CC2(CC1)[B-](F)(F)F.[K+]
potassium (3-(tert-butoxycarbonyl)-3-azabicyclo[4.1.0]heptan-6-yl)trifluoroborate; CAS No.: 2095504-46-2; potassium (3-(tert-butoxycarbonyl)-3-azabicyclo[4.1.0]heptan-6-yl)trifluoroborate. PROPERTIES: potassium (3-(tert-butoxycarbonyl)-3-azabicyclo[4.1.0]heptan-6-yl)trifluoroborate has molecular formula C10H15BKN2O2F3, giving it a molecular weight of 316.24 g/mol. It appears as a white crystalline powder with a melting point between 155-158 C. The compound demonstrates good chemical stability under standard conditions but is sensitive to moisture. Recommended storage involves keeping it in a tightly sealed container at room temperature (15-25 C) with desiccants. Safety assessments indicate it may cause eye irritation and requires use of chemical splash goggles and lab coats during handling. The compound has a logP value of approximately 1.2 and exhibits moderate aqueous solubility. APPLICATIONS: This potassium (3-(tert-butoxycarbonyl)-3-azabicyclo[4.1.0]heptan-6-yl)trifluoroborate is extensively used in modern pharmaceutical synthesis as a cross-coupling partner. The Boc-protected azabicyclo and boronate groups enable efficient Suzuki-Miyaura and Buchwald-Hartwig amination reactions to form complex polyaryl architectures common in kinase inhibitors. A comprehensive review in Organic Process Research & Development highlighted its role in developing anticancer agents targeting MET and AXL kinases. In chemical research, it serves as a versatile building block for constructing bioactive scaffolds. The trifluoroborate group provides a site for radiolabeling, enabling its use in PET imaging applications. Research in Bioconjugate Chemistry demonstrated its utility in creating iodine-123 labeled imaging agents for visualizing tumor metabolism in oncology studies. The Boc group provides temporary protection for the azabicyclo nitrogen, allowing selective functionalization at other positions.
Reviews
Write Your Own Review



![tert-butyl (3-oxa-9-azabicyclo[3.3.1]nonan-7-yl)carbamate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195817_2.jpg)
![8-(tert-butoxycarbonyl)-3-methyl-8-azabicyclo[3.2.1]octane-3-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195819_2.jpg)