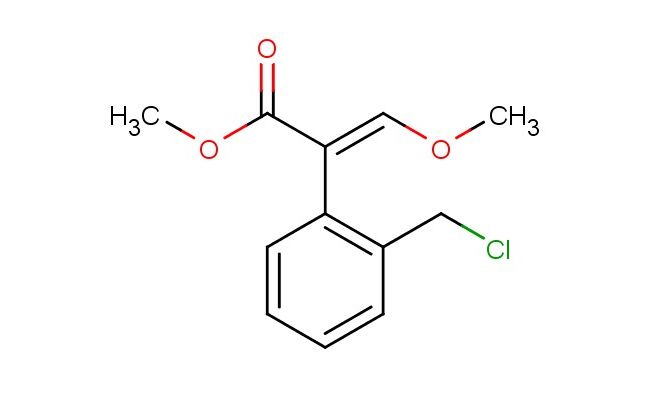
Methyl (E)-3-methoxy-2-(2-chloromethylphenyl)-2-propenoate
$250.00
CAS No.: 117428-51-0
Catalog No.: 200366
Purity: 95%
MF: C12H13ClO3
MW: 240.686
Storage: 2-8 degree Celsius
SMILES: CO/C=C(/C(=O)OC)\C1=C(C=CC=C1)CCl
Catalog No.: 200366
Purity: 95%
MF: C12H13ClO3
MW: 240.686
Storage: 2-8 degree Celsius
SMILES: CO/C=C(/C(=O)OC)\C1=C(C=CC=C1)CCl
For R&D use only. Not for human or animal use.
CAS NO.: 117428-51-0;Methyl (E)-3-methoxy-2-(2-chloromethylphenyl)-2-propenoate. PROPERTIES: This cinnamic acid derivative appears as orange-brown viscous liquid with characteristic ester fragrance, displaying moderate solubility in ethyl acetate and dichloromethane but forming emulsions in water. The Methyl (E)-3-methoxy-2-(2-chloromethylphenyl)-2-propenoate has molecular weight approximately 282.7 g/mol, combining alpha,beta-unsaturated ester with chloromethyl arene. Stability evaluation reveals tendency to undergo Diels-Alder reactions with electron-rich dienes, thus requiring storage at 2-8 degree Celsius in narrow-mouthed brown bottles. Personal protective measures should include butyl rubber gloves and full-face respirators when handling in bulk. Eye contact may cause permanent damage due to Michael addition potential, necessitating immediate rinsing and ophthalmology consultation. Thermal decomposition above 120 C may release toxic phosgene gas. Spill containment should utilize alkaline absorbent materials to neutralize any formed acids. APPLICATIONS: The Methyl (E)-3-methoxy-2-(2-chloromethylphenyl)-2-propenoate functions as a Michael acceptor in kinetic resolution processes, particularly valuable in chiral pharmaceutical synthesis. Its chloromethyl benzyl group enables post-condensation functionalization through nucleophilic aromatic substitution. The compound serves as a key intermediate in the preparation of beta-blockers and calcium channel antagonists. Additionally, it acts as a building block for fluorescent probes in bioimaging applications where the conjugated system provides Stokes shift advantages. In materials science, the compound undergoes thiol-ene click chemistry for creating patterned surfaces. Research laboratories employ it as a model compound for studying photocycloaddition reactions in organic photoredox catalysis.
Reviews
Write Your Own Review