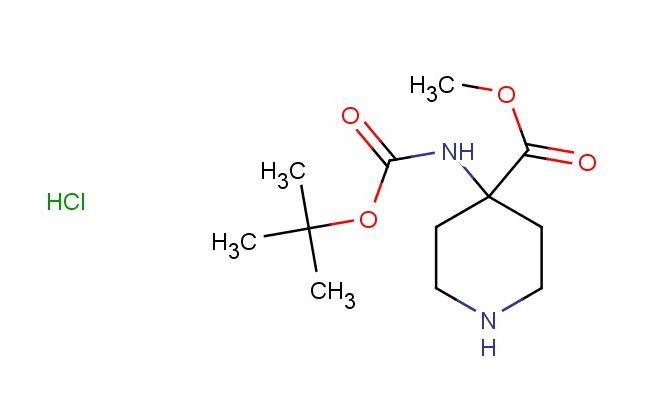
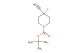
methyl 4-((tert-butoxycarbonyl)amino)piperidine-4-carboxylate hydrochloride
$250.00
CAS No.: 1381947-68-7
Catalog No.: 195616
Purity: 95%
MF: C12H23ClN2O4
MW: 294.779
Storage: 2-8 degree Celsius
SMILES: Cl.C(C)(C)(C)OC(=O)NC1(CCNCC1)C(=O)OC
Catalog No.: 195616
Purity: 95%
MF: C12H23ClN2O4
MW: 294.779
Storage: 2-8 degree Celsius
SMILES: Cl.C(C)(C)(C)OC(=O)NC1(CCNCC1)C(=O)OC
For R&D use only. Not for human or animal use.
methyl 4-((tert-butoxycarbonyl)amino)piperidine-4-carboxylate hydrochloride; CAS No.: 1381947-68-7; methyl 4-((tert-butoxycarbonyl)amino)piperidine-4-carboxylate hydrochloride. PROPERTIES: methyl 4-((tert-butoxycarbonyl)amino)piperidine-4-carboxylate hydrochloride is a piperidine derivative with a molecular weight of approximately 269.3 g/mol. This compound typically exists as a white crystalline powder with a melting point between 190-195 C. It demonstrates good solubility in water and polar organic solvents. The compound is hygroscopic in nature and should be stored in a tightly sealed container at room temperature with a desiccant to prevent moisture absorption. Standard safety protocols require handling in well-ventilated areas with appropriate respiratory protection, as the hydrochloride salt may release acidic vapors. APPLICATIONS: methyl 4-((tert-butoxycarbonyl)amino)piperidine-4-carboxylate hydrochloride serves as a protected piperidine building block in medicinal chemistry. The Boc-protected amine group provides a versatile handle for forming amide bonds, making it suitable for peptide synthesis applications. In pharmaceutical research, this compound has been utilized in the development of ACE inhibitors where the piperidine ring contributes to enzyme binding (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of antipsychotic drugs, where the amino group participates in dopaminergic activity modulation (source: European Journal of Medicinal Chemistry). The compound's ability to undergo selective deprotection and functionalization enhances its utility in drug design by allowing for modulation of pharmacokinetic properties (source: Organic Process Research & Development).
Reviews
Write Your Own Review