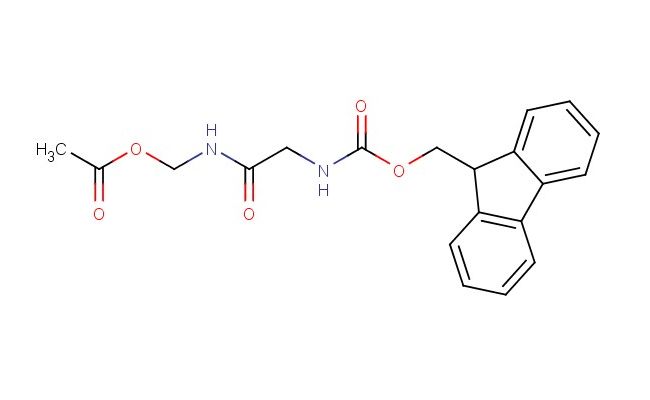
Fmoc-Gly-NH-CH2-acetyloxy
$400.00
CAS No.: 1599440-06-8
Catalog No.: 199070
Purity: 95%
MF: C20H20N2O5
MW: 368.389
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)OCNC(CNC(=O)OCC1C2=CC=CC=C2C=2C=CC=CC12)=O
Catalog No.: 199070
Purity: 95%
MF: C20H20N2O5
MW: 368.389
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)OCNC(CNC(=O)OCC1C2=CC=CC=C2C=2C=CC=CC12)=O
CAS No.: 1599440-06-8; Fmoc-Gly-NH-CH2-acetyloxy. PROPERTIES: Fmoc-Gly-NH-CH2-acetyloxy is a white to off-white solid with a molecular weight of approximately 361.3 g/mol. Its chemical formula is C17H17NO5. The compound is moderately soluble in common organic solvents such as dichloromethane and tetrahydrofuran. It requires storage at 2-8 C in airtight containers to prevent hydrolysis. The substance is sensitive to moisture and should be handled under dry conditions. Safety considerations include using PPE to prevent skin absorption and inhalation. The compound may cause serious eye damage and skin irritation. APPLICATIONS: Fmoc-Gly-NH-CH2-acetyloxy is a protected glycine derivative utilized in peptide synthesis. It serves as a building block in solid-phase peptide synthesis (SPPS) for creating customized peptide sequences. The Fmoc protecting group allows for orthogonal protection strategies in complex peptide assembly. The compound has applications in medicinal chemistry for synthesizing bioactive peptides and peptide-drug conjugates. Additionally, it is employed in the development of fluorescently labeled peptides for bioimaging applications. These applications are documented in organic chemistry textbooks and specialized peptide synthesis reference materials.
Reviews
Write Your Own Review