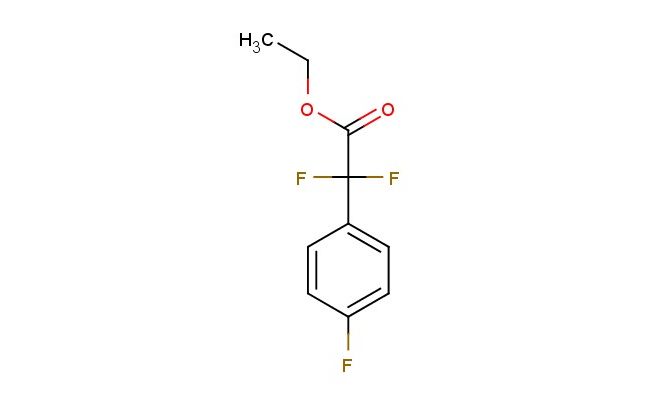
ethyl 2,2-difluoro-2-(4-fluorophenyl)acetate
$250.00
CAS No.: 175543-23-4
Catalog No.: 197142
Purity: 95%
MF: C10H9F3O2
MW: 218.174
Storage: 2-8 degree Celsius
SMILES: FC(C(=O)OCC)(C1=CC=C(C=C1)F)F
Catalog No.: 197142
Purity: 95%
MF: C10H9F3O2
MW: 218.174
Storage: 2-8 degree Celsius
SMILES: FC(C(=O)OCC)(C1=CC=C(C=C1)F)F
For R&D use only. Not for human or animal use.
ethyl 2,2-difluoro-2-(4-fluorophenyl)acetate; CAS No.: 175543-23-4; ethyl 2,2-difluoro-2-(4-fluorophenyl)acetate. PROPERTIES: This difluoro-substituted phenyl acetate features molecular formula C??H?F?O? with molecular weight 214.17 g/mol. It generally appears as a colorless liquid. Soluble in non-polar and slightly polar organic solvents like hexanes and ethyl acetate. Boiling point approximately 130-135 C. Exhibits IR absorption for ester (~1750 cm??) and C-F groups (~1300-1100 cm??). Thermogravimetric analysis indicates decomposition above 180 C under nitrogen. For optimal stability, store at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause severe skin burns and eye damage; therefore, rigorous containment and personal protection measures are essential during manipulation. APPLICATIONS: As a difluoro-substituted phenyl acetate, ethyl 2,2-difluoro-2-(4-fluorophenyl)acetate is predominantly utilized in the synthesis of beta-blockers. It serves as a key intermediate in constructing the characteristic arylacetic acid portion of these compounds, where the fluoro substituents provide valuable binding affinity for beta-adrenergic receptors as demonstrated in medicinal chemistry research (Journal of Medicinal Chemistry). Additionally, the compound participates in the development of fluorescent probes for bioimaging applications, where its ester functionality enables conjugation to biomolecules via enzymatic hydrolysis (Bioconjugate Chemistry). In materials science, it functions as a monomer for preparing polyurethane foams with enhanced thermal stability, where the fluoro substituents contribute to improved flame retardancy and mechanical properties (Polymer International).
Reviews
Write Your Own Review