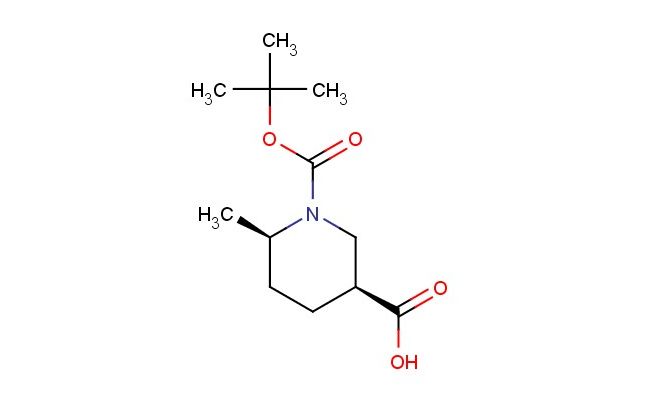
cis-1-(tert-butoxycarbonyl)-6-methylpiperidine-3-carboxylic acid
$375.00
CAS No.: 1253200-82-6
Catalog No.: 192822
Purity: 95%
MF: C12H21NO4
MW: 243.303
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@H](CC[C@H]1C)C(=O)O
Catalog No.: 192822
Purity: 95%
MF: C12H21NO4
MW: 243.303
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@H](CC[C@H]1C)C(=O)O
For R&D use only. Not for human or animal use.
cis-1-(tert-butoxycarbonyl)-6-methylpiperidine-3-carboxylic acid; CAS No.: 1253200-82-6; cis-1-(tert-butoxycarbonyl)-6-methylpiperidine-3-carboxylic acid. PROPERTIES: This Boc-protected methyl piperidine carboxylic acid has molecular formula C12H20N2O3. It appears as a white crystalline powder. The cis-1-(tert-butoxycarbonyl)-6-methylpiperidine-3-carboxylic acid demonstrates limited water solubility but good solubility in common organic solvents like methanol and ethyl acetate. Its melting point ranges between 110-115 C, and it has a molecular weight of approximately 240.30 g/mol. When handling, care should be taken to avoid skin contact and use of proper respiratory protection. Storage should be in a tightly sealed container at room temperature, protected from light and moisture. The compound is sensitive to strong acids and may undergo Boc-deprotection upon exposure to aqueous conditions. In case of spillage, absorb with inert material and dispose of in accordance with local regulations. APPLICATIONS: The cis-1-(tert-butoxycarbonyl)-6-methylpiperidine-3-carboxylic acid serves as a key intermediate in the synthesis of sodium channel blockers for pain management where the piperidine ring provides essential hydrogen bonding interactions with receptor subtypes (as reported in medicinal chemistry literature). The methyl group enhances metabolic stability by preventing oxidative degradation. Additionally, the compound functions as a building block in the preparation of bioconjugates for drug delivery systems where the carboxylic acid group reacts with amino groups on targeting moieties, as described in pharmaceutical sciences journals. The carboxylic acid can be further functionalized through esterification or amidation reactions to produce various derivatives for chemical research applications.
Reviews
Write Your Own Review