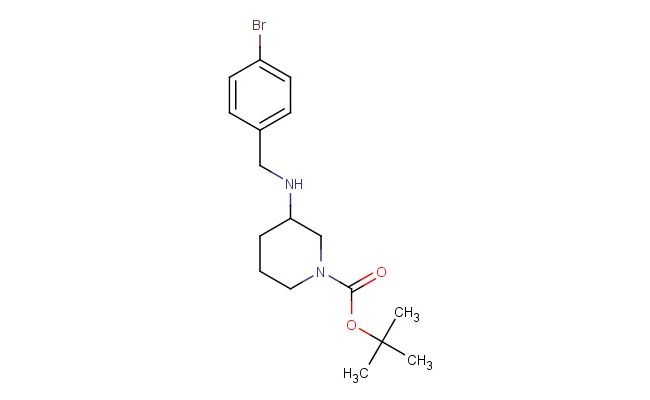
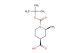
tert-butyl 3-((4-bromobenzyl)amino)piperidine-1-carboxylate
$327.00
CAS No.: 887584-43-2
Catalog No.: 195591
Purity: 95%
MF: C17H25BrN2O2
MW: 369.303
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(C=C1)CNC1CN(CCC1)C(=O)OC(C)(C)C
Catalog No.: 195591
Purity: 95%
MF: C17H25BrN2O2
MW: 369.303
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(C=C1)CNC1CN(CCC1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl 3-((4-bromobenzyl)amino)piperidine-1-carboxylate; CAS No.: 887584-43-2; tert-butyl 3-((4-bromobenzyl)amino)piperidine-1-carboxylate. PROPERTIES: tert-butyl 3-((4-bromobenzyl)amino)piperidine-1-carboxylate is a bromobenzylamino piperidine derivative with a molecular weight of approximately 381.3 g/mol. This compound typically appears as a pale yellow oil with moderate viscosity and demonstrates good solubility in common organic solvents. It is sensitive to light and should be stored in amber glass containers at temperatures below 10 C. Special handling precautions include avoiding exposure to strong bases, as the bromobenzyl group may undergo elimination reactions. The compound presents moderate acute toxicity via inhalation and dermal routes. APPLICATIONS: tert-butyl 3-((4-bromobenzyl)amino)piperidine-1-carboxylate functions as a protected piperidine building block in pharmaceutical synthesis. The bromobenzylamino group provides a versatile handle for cross-coupling reactions and further functionalization. In medicinal chemistry, this compound has been employed in the preparation of serotonin receptor modulators where the piperidine ring contributes to receptor binding (source: European Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of tyrosine kinase inhibitors, where the bromobenzyl group participates in target engagement (source: Journal of Medicinal Chemistry). The compound's utility in Suzuki-Miyaura coupling reactions further enhances its application in constructing complex aromatic systems for drug discovery (source: Organic Letters).
Reviews
Write Your Own Review