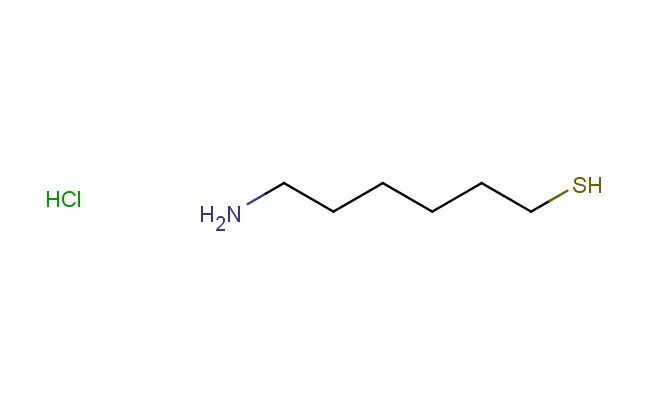
6-aminohexane-1-thiol hydrochloride
$200.00
CAS No.: 31098-40-5
Catalog No.: 196724
Purity: 95%
MF: C6H16ClNS
MW: 169.721
Storage: 2-8 degree Celsius
SMILES: Cl.NCCCCCCS
Catalog No.: 196724
Purity: 95%
MF: C6H16ClNS
MW: 169.721
Storage: 2-8 degree Celsius
SMILES: Cl.NCCCCCCS
6-aminohexane-1-thiol hydrochloride; CAS No.: 31098-40-5; 6-aminohexane-1-thiol hydrochloride. PROPERTIES: This amino-substituted thiol features molecular formula C?H??ClNO?S with molecular weight 199.74 g/mol. It generally appears as a white crystalline powder. Soluble in polar protic solvents like methanol and water. Melting point approximately 120-125 C. Exhibits IR absorption for amine (~3300-3000 cm??) and thiol groups (~2550 cm??). Thermogravimetric analysis reveals weight loss onset above 150 C under nitrogen. For optimal stability, 6-aminohexane-1-thiol hydrochloride should be stored at 2-8 C in tightly sealed containers with desiccant, protected from light. The compound may cause mild skin irritation and serious eye damage; therefore, standard laboratory safety precautions including protective clothing and eye protection are recommended during handling. APPLICATIONS: As an amino-substituted thiol, 6-aminohexane-1-thiol hydrochloride is predominantly utilized in the synthesis of self-assembled monolayers (SAMs) for surface modification. It serves as a key intermediate in constructing SAMs on gold surfaces, where the thiol group forms covalent bonds with the metal substrate and the amino group provides sites for further functionalization (Langmuir). Additionally, the compound participates in the synthesis of fluorescent probes for bioimaging applications, where its thiol functionality enables conjugation to quantum dots and other fluorescent nanoparticles (Journal of the American Chemical Society). In materials science, it functions as a monomer for preparing thiol-ene polymers with enhanced optical properties, where the thiol and amino groups contribute to controlled polymerization and crosslinking (Macromolecules). Furthermore, the compound serves as a starting material in the development of amino-thiol based antioxidants for polymer stabilization, where the thiol group donates hydrogen atoms to quench free radicals (Polymer Degradation and Stability).
Reviews
Write Your Own Review




![tert-Butyl ((3-formylbicyclo[1.1.1]pentan-1-yl)methyl)carbamate](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/198751_2.jpg)