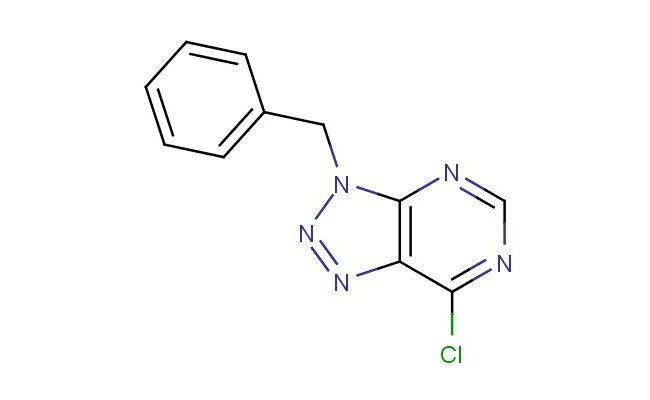
3-benzyl-7-chloro-3H-[1,2,3]triazolo[4,5-d]pyrimidine
$250.00
CAS No.: 21410-06-0
Catalog No.: 196125
Purity: 95%
MF: C11H8ClN5
MW: 245.673
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)N1N=NC2=C1N=CN=C2Cl
Catalog No.: 196125
Purity: 95%
MF: C11H8ClN5
MW: 245.673
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)N1N=NC2=C1N=CN=C2Cl
For R&D use only. Not for human or animal use.
3-benzyl-7-chloro-3H-[1,2,3]triazolo[4,5-d]pyrimidine; CAS No.: 21410-06-0; 3-benzyl-7-chloro-3H-[1,2,3]triazolo[4,5-d]pyrimidine. PROPERTIES: This white to pale yellow crystalline solid has a molecular formula of C12H9ClN4 and a molecular weight of approximately 248.68 g/mol. It exhibits low water solubility but dissolves in DMSO, DMF, and ethanol. The compound is stable under normal laboratory conditions but should be stored in a tightly sealed container at room temperature. Thermogravimetric analysis shows decomposition starting at 250 C. Safety precautions include using chemical-resistant gloves, safety goggles, and working in a well-ventilated area. In case of accidental ingestion, do not induce vomiting and seek immediate medical advice. Avoid release to the environment as chlorinated heterocycles may affect aquatic organisms. APPLICATIONS: 3-benzyl-7-chloro-3H-[1,2,3]triazolo[4,5-d]pyrimidine functions as a versatile intermediate in nucleic acid chemistry and pharmaceutical synthesis. Its triazolopyrimidine core resembles purine bases, making it suitable for oligonucleotide modification studies. The benzyl group provides steric and electronic effects useful in modulating binding interactions with target proteins. Research groups employ it in the development of adenosine receptor antagonists for cardiovascular applications. Academic institutions utilize it in teaching nucleoside chemistry and heterocyclic synthesis methodologies. Industrial applications include its use as a building block in agrochemical development for novel fungicide candidates targeting nucleic acid synthesis. Recent studies in Nucleic Acids Research highlight its application in developing modified oligonucleotides with enhanced stability and binding affinity. Additionally, it serves as a starting material for radiolabeled compounds used in receptor autoradiography studies. The compound's ability to form hydrogen bonds makes it suitable for crystallographic studies of protein-ligand complexes.
Reviews
Write Your Own Review



![8-bromo-5-(methylthio)-[1,2,4]triazolo[4,3-c]pyrimidine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/8/181958_1.jpg)
![7-chloro-5-methyl-[1,2,4]triazolo[1,5-a]pyrimidine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196127_2.jpg)