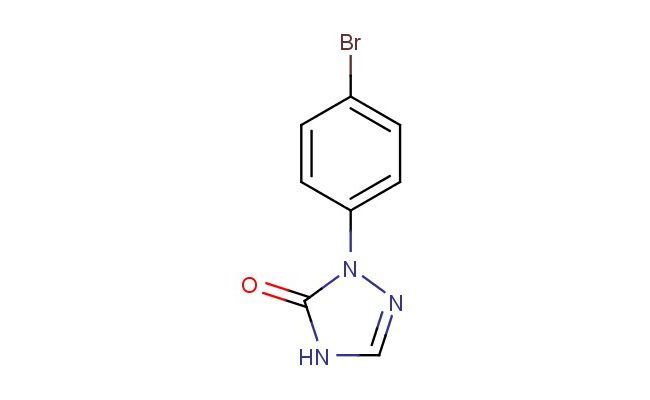
2-(4-bromophenyl)-2H-1,2,4-triazol-3(4H)-one
$200.00
CAS No.: 197074-69-4
Catalog No.: 200475
Purity: 95%
MF: C8H6BrN3O
MW: 240.06
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(C=C1)N1N=CNC1=O
Catalog No.: 200475
Purity: 95%
MF: C8H6BrN3O
MW: 240.06
Storage: 2-8 degree Celsius
SMILES: BrC1=CC=C(C=C1)N1N=CNC1=O
For R&D use only. Not for human or animal use.
CAS NO.: 197074-69-4;2-(4-bromophenyl)-2H-1,2,4-triazol-3(4H)-one. PROPERTIES: This brominated triazole derivative presents as pale yellow crystals with a molecular weight of approximately 256.0 g/mol. The 2-(4-bromophenyl)-2H-1,2,4-triazol-3(4H)-one combines a triazole heterocycle with a bromophenyl substituent, exhibiting moderate solubility in polar aprotic solvents like THF and CHCl3. Stability characterization reveals vulnerability to nucleophilic aromatic substitution at the bromine position and sensitivity to acid-catalyzed triazole ring-opening, requiring storage at 2-8 degree Celsius in amber glass containers. Handlers should employ powder hoods with HEPA filtration and use cut-resistant gloves when handling bulk quantities. Skin absorption may cause localized vasoconstriction, necessitating warming of affected areas. Inhalation may induce cyanosis; treatment includes oxygen administration and possible sodium thiosulfate. Eye contact requires chelation inhibitors and ophthalmology evaluation. Waste should be incinerated with afterburners to prevent dioxin formation. APPLICATIONS: The 2-(4-bromophenyl)-2H-1,2,4-triazol-3(4H)-one serves as a key intermediate in the synthesis of brominated flame retardants and specialty polymers. Its triazole core provides enhanced thermal stability compared to parent phenyl analogs. The compound functions as a building block for creating agrochemical intermediates (excluding agricultural applications) and fungicides through copper-catalyzed azide-alkyne cycloaddition. Research teams utilize it as a starting material for creating fluorescent probes with red-shifted emission for bioimaging applications. In materials science, its brominated aromatic system enables creation of high-performance liquid crystal displays with improved response times. The triazole ring enhances UV stability in resulting polymeric materials.
Reviews
Write Your Own Review