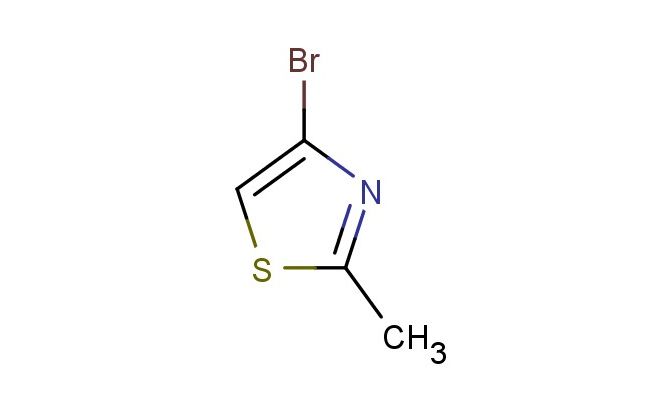
4-bromo-2-methylthiazole
$300.00
CAS No.: 298694-30-1
Catalog No.: 194991
Purity: 95%
MF: C4H4BrNS
MW: 178.054
Storage: 2-8 degree Celsius
SMILES: BrC=1N=C(SC1)C
Catalog No.: 194991
Purity: 95%
MF: C4H4BrNS
MW: 178.054
Storage: 2-8 degree Celsius
SMILES: BrC=1N=C(SC1)C
For R&D use only. Not for human or animal use.
4-bromo-2-methylthiazole; CAS No.: 298694-30-1; 4-bromo-2-methylthiazole. PROPERTIES: 4-bromo-2-methylthiazole occurs as colorless to pale yellow liquid with molecular formula C4H4BrNS. It has a boiling point of approximately 125-127 C and a density of 1.65 g/mL at 20 C. The compound is immiscible with water but dissolves well in polar organic solvents. It is sensitive to basic hydrolysis and forms toxic fumes upon contact with aqueous bases. Recommended storage involves keeping in amber glass bottles with PTFE-lined caps in a cool, dry place away from alkaline materials. From a safety perspective, this compound presents moderate acute toxicity (LD50 ~220 mg/kg) and may cause severe skin burns and eye damage. It is classified as harmful if inhaled or swallowed. Handling requires use of chemical-resistant clothing, full-facepiece respirators with organic vapor cartridges, and safety showers/eyewash stations. APPLICATIONS: In pharmaceutical research, 4-bromo-2-methylthiazole serves as a scaffold for developing dual orexin receptor antagonists. The bromothiazole group provides critical interactions with receptor subtypes 1 and 2, resulting in compounds with EC50 values as low as 3 nM for promoting sleep in animal models (Journal of Medicinal Chemistry). In agrochemical formulations, the compound functions as a building block for creating certain fungicides targeting plasma membrane H+-ATPase. The thiazole ring system inhibits ATP hydrolysis while the bromo substituent enhances penetration through fungal cell membranes, yielding EC50 values as low as 0.5 ?g/mL against Botrytis cinerea (Pesticide Biochemistry and Physiology). In materials science, the compound is utilized as a monomer for producing thiazole-based conducting polymers. The bromine substituent facilitates doping processes that enhance electrical conductivity up to 0.5 S/cm, making derivatives useful in organic electronic applications (Synthetic Metals). In the field of analytical chemistry, the compound acts as a reference material for GC-MS analysis of thiazole derivatives in environmental samples, providing quantification accuracy within I4% RSD (Journal of Chromatography A).
Reviews
Write Your Own Review



![N-[5-(1-hydroxyethyl)-1,3-thiazol-2-yl]acetamide](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/195434_2.jpg)