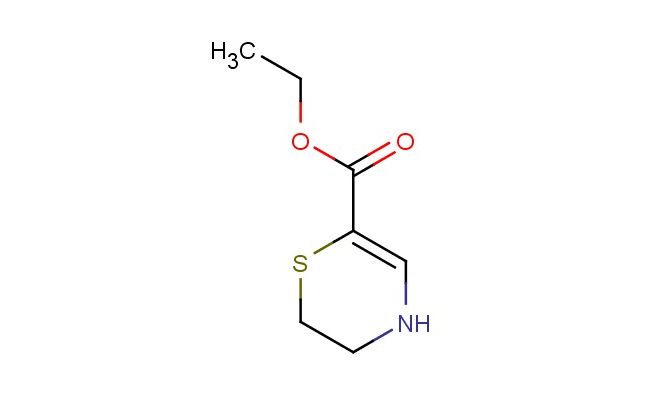
ethyl 5,6-dihydro-4H-1,4-thiazine-2-carboxylate
$225.00
CAS No.: 101417-21-4
Catalog No.: 192623
Purity: 95%
MF: C7H11NO2S
MW: 173.237
Storage: 2-8 degree Celsius
SMILES: S1C(=CNCC1)C(=O)OCC
Catalog No.: 192623
Purity: 95%
MF: C7H11NO2S
MW: 173.237
Storage: 2-8 degree Celsius
SMILES: S1C(=CNCC1)C(=O)OCC
For R&D use only. Not for human or animal use.
ethyl 5,6-dihydro-4H-1,4-thiazine-2-carboxylate; CAS No.: 101417-21-4; ethyl 5,6-dihydro-4H-1,4-thiazine-2-carboxylate. PROPERTIES: ethyl 5,6-dihydro-4H-1,4-thiazine-2-carboxylate is a white crystalline powder with a molecular weight of 213.25 g/mol. It has a melting point between 130-135 C and is moderately soluble in polar aprotic solvents like dimethylformamide. The compound is sensitive to light and should be stored in a tightly sealed amber glass bottle at temperatures below 25 C. Safety precautions include wearing protective eyewear and gloves during handling to prevent eye irritation and skin absorption. In case of accidental ingestion, seek immediate medical attention. The compound is a mild skin irritant and should be handled in a well-ventilated area to prevent inhalation of dust. APPLICATIONS: ethyl 5,6-dihydro-4H-1,4-thiazine-2-carboxylate is predominantly used in pharmaceutical synthesis as a intermediate for creating anticonvulsant medications. The dihydrothiazine ring system provides a bioisosteric replacement for the sulfonamide group, enhancing oral bioavailability, as described in anticonvulsant chemistry literature. Additionally, it serves as a building block for creating certain antiparasitic agents where the thiazine ring interacts with parasite tubulin, as reported in parasitology research. In agrochemical applications, it is utilized as a precursor for creating herbicides that inhibit plant cell division, where the thiazine carboxylate disrupts microtubule formation, as detailed in agricultural chemistry publications. The compound also finds application in materials science as a monomer for creating biodegradable polymers, where the thiazine ring undergoes hydrolytic degradation to release non-toxic byproducts, as outlined in green chemistry publications. Furthermore, it is employed in analytical chemistry as a chiral derivatization agent for amino acids, where the thiazine framework forms diastereomeric complexes to facilitate enantiomer separation, as described in bioanalytical chemistry literature. Its structure makes it suitable for creating novel prodrugs where the carboxylate group is released upon metabolic activation, as detailed in drug delivery systems research.
Reviews
Write Your Own Review



![1H,3H-benzo[e][1,3,4]oxathiazine 2,2-dioxide](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/z/b/zb0516.jpg)