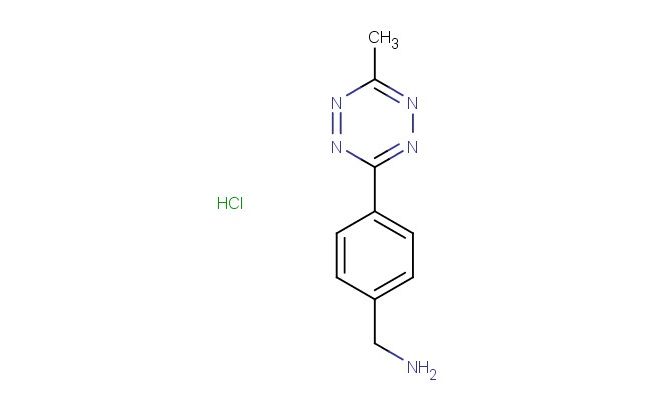
(4-(6-methyl-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride
$300.00
CAS No.: 1596117-29-1
Catalog No.: TQP1157
Purity: 95%
MF: C10H12ClN5
MW: 237.694
Storage: 2-8 degree Celsius
SMILES: Cl.CC1=NN=C(N=N1)C1=CC=C(C=C1)CN
Catalog No.: TQP1157
Purity: 95%
MF: C10H12ClN5
MW: 237.694
Storage: 2-8 degree Celsius
SMILES: Cl.CC1=NN=C(N=N1)C1=CC=C(C=C1)CN
For R&D use only. Not for human or animal use.
CAS NO.: 1596117-29-1; (4-(6-methyl-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride. PROPERTIES: (4-(6-methyl-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride appears as a white to off-white crystalline powder with characteristic amine salt properties. The compound contains a tetrazine ring system conjugated to an aniline moiety, creating a molecule with interesting electronic characteristics. Its molecular formula is C12H12N6 HCl, resulting in a molecular weight of approximately 300.72 g/mol. The substance exhibits moderate hygroscopicity and should be protected from excessive moisture. Thermally stable up to 130 C, decomposition at higher temperatures may release nitrogen oxides and other toxic fumes. (4-(6-methyl-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride must be stored between 2-8 degree Celsius in tightly sealed containers. When handling, standard laboratory precautions including eye protection and gloves are recommended. The compound may cause eye irritation and should be handled in well-ventilated areas. In case of skin contact, washing with soap and water is advised. The tetrazine functionality requires particular attention due to its potential reactivity with electrophilic agents. APPLICATIONS: (4-(6-methyl-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride serves as a versatile intermediate in bioconjugation chemistry. The tetrazine ring enables inverse electron-demand Diels-Alder reactions with trans-cyclooctene moieties, facilitating click chemistry applications in biological systems. Researchers utilize this compound for site-specific protein labeling and imaging studies. The methyl-substituted tetrazine enhances reactivity while the aniline group provides opportunities for further functionalization. Medicinal chemists employ (4-(6-methyl-1,2,4,5-tetrazin-3-yl)phenyl)methanamine hydrochloride derivatives in prodrug development, where the tetrazine group reacts with endogenous biomolecules to release active agents. The compound's utility extends to bioorthogonal ligation reactions in living cells, enabling real-time tracking of biomolecular interactions. Proper storage at 2-8 C maintains the reactivity of the tetrazine ring system, ensuring reliable performance in chemical and biological applications.
Reviews
Write Your Own Review