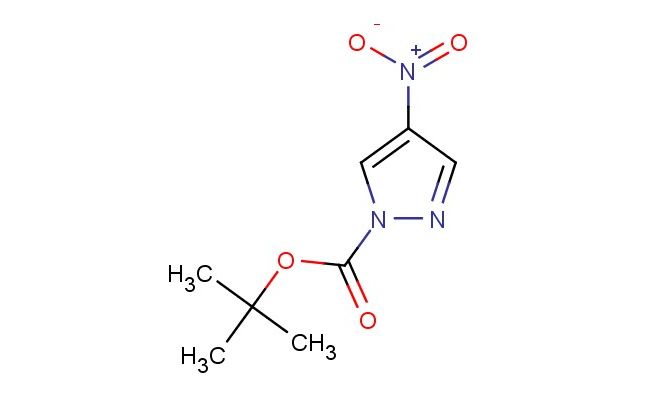
tert-butyl 4-nitro-1H-pyrazole-1-carboxylate
$250.00
CAS No.: 1018446-96-2
Catalog No.: 192864
Purity: 95%
MF: C8H11N3O4
MW: 213.193
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C=1C=NN(C1)C(=O)OC(C)(C)C
Catalog No.: 192864
Purity: 95%
MF: C8H11N3O4
MW: 213.193
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C=1C=NN(C1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl 4-nitro-1H-pyrazole-1-carboxylate; CAS No.: 1018446-96-2; tert-butyl 4-nitro-1H-pyrazole-1-carboxylate. PROPERTIES: This compound appears as a pale yellow crystalline solid with molecular formula C9H11N3O4 and a molecular weight of 229.20 g/mol. It demonstrates a melting point in the range of 82-86 C and shows moderate solubility in common organic solvents like ethyl acetate and dichloromethane. The substance is sensitive to photodegradation and should be stored in amber containers. Recommended storage conditions include maintaining in tightly sealed containers at temperatures below 20 C, protected from light and moisture. When handling, standard laboratory safety protocols should be followed, including the use of protective gloves and eye wear, as tert-butyl 4-nitro-1H-pyrazole-1-carboxylate may cause skin sensitization and eye irritation. The nitro group requires careful handling in reducing environments. APPLICATIONS: In the field of medicinal chemistry, tert-butyl 4-nitro-1H-pyrazole-1-carboxylate serves as a key intermediate for synthesizing anti-inflammatory agents, as documented in pharmaceutical research focusing on pyrazole derivatives. Its nitro substituent enables reduction to generate amino groups for further functionalization in drug discovery. Additionally, this compound functions as a building block for preparing agricultural fungicides through nucleophilic aromatic substitution reactions. Academic research has explored its utility in the development of nitric oxide-releasing molecules for cardiovascular applications, as reported in biochemical journals. The tert-butyl ester group also facilitates controlled hydrolysis in biological systems, making it valuable for prodrug strategies in drug delivery systems, as evidenced by studies in pharmaceutical sciences literature.
Reviews
Write Your Own Review