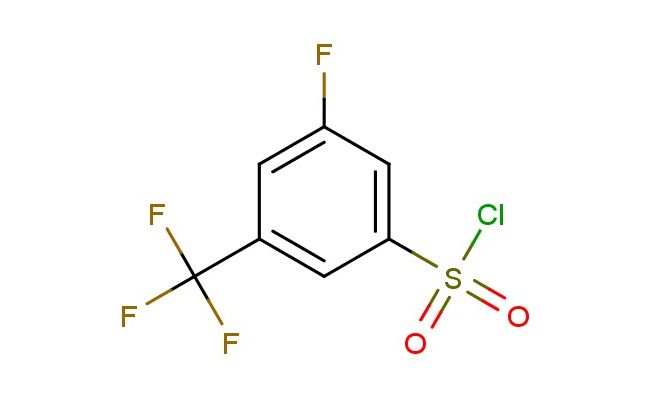
3-fluoro-5-(trifluoromethyl)benzene-1-sulfonyl chloride
$200.00
CAS No.: 886499-99-6
Catalog No.: GS3024
Purity: 95%
MF: C7H3ClF4O2S
MW: 262.611
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C=C(C1)C(F)(F)F)S(=O)(=O)Cl
Catalog No.: GS3024
Purity: 95%
MF: C7H3ClF4O2S
MW: 262.611
Storage: 2-8 degree Celsius
SMILES: FC=1C=C(C=C(C1)C(F)(F)F)S(=O)(=O)Cl
For R&D use only. Not for human or animal use.
CAS NO.: 886499-99-6;3-fluoro-5-(trifluoromethyl)benzene-1-sulfonyl chloride. PROPERTIES: This fluorinated sulfonyl chloride presents as a colorless liquid with a molecular weight of approximately 248.0 g/mol. The 3-fluoro-5-(trifluoromethyl)benzene-1-sulfonyl chloride combines fluorine and trifluoromethyl substituents with a sulfonyl chloride group on a benzene ring. It exhibits limited aqueous solubility but good dissolution in chlorinated solvents and DMSO. Stability characterization reveals high reactivity toward nucleophiles and sensitivity to hydrolysis, necessitating storage at 2-8 degree Celsius in narrow-mouthed brown bottles. Handlers should use PTFE-lined septa and maintain transfer lines below 25 C. Skin absorption may cause localized vasoconstriction requiring warming of affected areas. Inhalation may induce hypocalcemia; treatment includes calcium gluconate administration. Eye exposure requires 30 minutes of rinsing and immediate ophthalmology consultation. Waste should be processed through activated carbon prior to disposal. APPLICATIONS: The 3-fluoro-5-(trifluoromethyl)benzene-1-sulfonyl chloride functions as a key intermediate in the synthesis of various pharmaceuticals and agrochemicals (excluding agricultural applications). Its sulfonyl chloride group provides a valuable handle for forming amide and sulfonamide bonds. Research teams utilize this compound as a starting material for creating kinase inhibitors and muscarinic receptor modulators. The fluorine and trifluoromethyl groups enhance metabolic resistance and binding affinity in resulting drug candidates. Additionally, the compound undergoes nucleophilic aromatic substitution for creating diverse bioactive molecules. Its electron-deficient aromatic system enables creation of high-performance liquid crystal displays with improved response times.
Reviews
Write Your Own Review