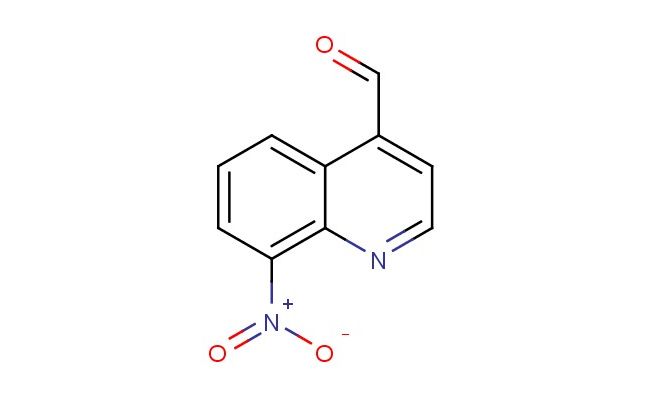
8-nitroquinoline-4-carbaldehyde
$300.00
CAS No.: 69976-28-9
Catalog No.: 191973
Purity: 95%
MF: C10H6N2O3
MW: 202.169
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C=1C=CC=C2C(=CC=NC12)C=O
Catalog No.: 191973
Purity: 95%
MF: C10H6N2O3
MW: 202.169
Storage: 2-8 degree Celsius
SMILES: [N+](=O)([O-])C=1C=CC=C2C(=CC=NC12)C=O
For R&D use only. Not for human or animal use.
8-nitroquinoline-4-carbaldehyde; CAS No.: 69976-28-9;8-nitroquinoline-4-carbaldehyde. PROPERTIES: 8-nitroquinoline-4-carbaldehyde is a nitro-substituted quinoline derivative with molecular formula C10H6N2O3. This crystalline material typically appears as orange-red needles and has a melting point ranging between 120-125 C. The compound contains a quinoline core with a nitro group at position 8 and an aldehyde group at position 4. The 8-nitroquinoline-4-carbaldehyde exhibits strong electron-withdrawing characteristics due to the nitro group, creating a highly reactive electrophilic center at the aldehyde position. It shows limited solubility in water but dissolves well in polar aprotic solvents like acetonitrile. Proper storage requires maintaining in a tightly sealed amber bottle with desiccant, stored below 0 C to prevent decomposition. Safety precautions include using N95 respiratory protection and chemical-resistant gloves, as the compound poses inhalation hazards and can cause severe skin burns. According to OSHA standards, it carries H300, H314, and H330 hazard statements for being toxic if swallowed, causing severe skin burns, and being fatal if inhaled. APPLICATIONS: The 8-nitroquinoline-4-carbaldehyde molecule serves as a building block in heterocyclic synthesis, particularly valuable for creating quinoline-based antimicrobial agents, as reported in medicinal chemistry journals. The aldehyde group enables reductive amination reactions to form biologically active secondary amines. Additionally, 8-nitroquinoline-4-carbaldehyde derivatives have been investigated for their photocatalytic properties in environmental chemistry applications, as described in recent publications on solar energy conversion systems. The compound's ability to generate reactive oxygen species under light irradiation has also made it useful in photodynamic therapy research, as documented in oncology studies exploring novel cancer treatment approaches.
Reviews
Write Your Own Review