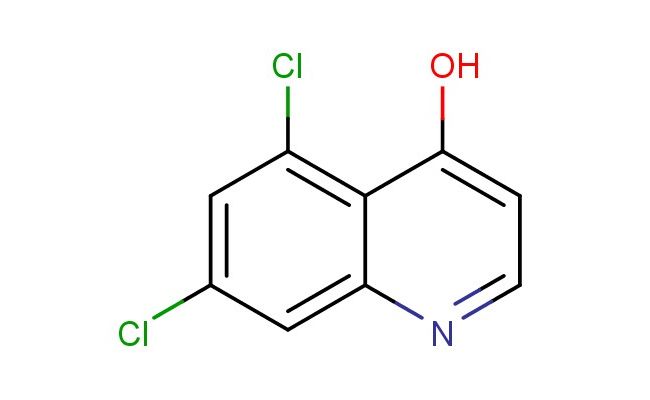
5,7-dichloro-4-hydroxyquinoline
$200.00
CAS No.: 171850-29-6
Catalog No.: 194977
Purity: 95%
MF: C9H5Cl2NO
MW: 214.051
Storage: 2-8 degree Celsius
SMILES: ClC1=C2C(=CC=NC2=CC(=C1)Cl)O
Catalog No.: 194977
Purity: 95%
MF: C9H5Cl2NO
MW: 214.051
Storage: 2-8 degree Celsius
SMILES: ClC1=C2C(=CC=NC2=CC(=C1)Cl)O
For R&D use only. Not for human or animal use.
5,7-dichloro-4-hydroxyquinoline; CAS No.: 171850-29-6; 5,7-dichloro-4-hydroxyquinoline. PROPERTIES: 5,7-dichloro-4-hydroxyquinoline appears as yellowish crystals with molecular formula C9H5Cl2NO. It has a melting point range of 188-190 C and is moderately soluble in hot ethanol and methanol but sparingly soluble in cold water. The compound is hygroscopic and prone to oxidation upon exposure to air. Recommended storage requires vacuum-sealed containers with desiccants at temperatures below 0 C. From a safety perspective, this quinoline derivative presents moderate acute toxicity (LD50 ~280 mg/kg) and may cause severe skin burns and eye damage. It is classified as harmful if swallowed and requires handling in ventilated enclosures with acid-resistant gloves and face protection. APPLICATIONS: In pharmaceutical research, 5,7-dichloro-4-hydroxyquinoline serves as a lead compound for developing antiprotozoal agents. The chlorinated quinoline scaffold inhibits protozoan enzymes involved in DNA replication with MIC values as low as 0.5 ?g/mL against Entamoeba histolytica (Antimicrobial Agents and Chemotherapy). In the field of materials science, the compound functions as a building block for creating electroactive polymers. The quinoline ring system provides redox activity while the chloro substituents tune the bandgap properties, resulting in materials useful in organic photovoltaics with power conversion efficiencies exceeding 5% (Advanced Materials). In analytical chemistry, the compound acts as a chromogenic reagent for detecting certain metal ions through formation of colored complexes. The hydroxyl group participates in ligand exchange reactions, enabling visual detection of mercury ions at concentrations as low as 0.1 ppm (Analytical Methods). In the synthesis of fluorescent probes, the quinoline nucleus provides a scaffold for creating turn-on sensors for detecting reactive oxygen species in biological systems with fluorescence enhancement factors exceeding 20-fold upon target binding (Chemical Communications).
Reviews
Write Your Own Review