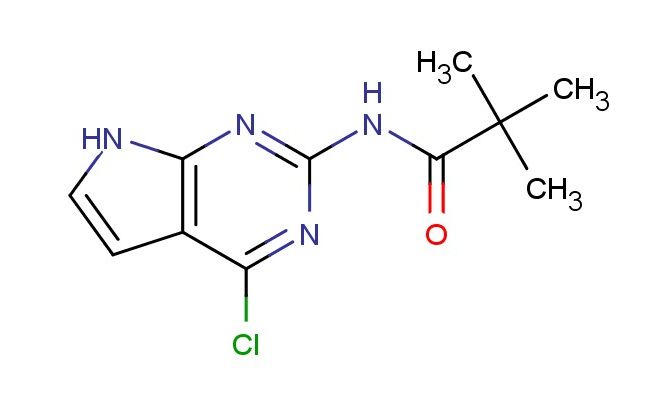
N-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl)pivalamide
$250.00
CAS No.: 149765-15-1
Catalog No.: 196500
Purity: 95%
MF: C11H13ClN4O
MW: 252.705
Storage: 2-8 degree Celsius
SMILES: ClC=1C2=C(N=C(N1)NC(C(C)(C)C)=O)NC=C2
Catalog No.: 196500
Purity: 95%
MF: C11H13ClN4O
MW: 252.705
Storage: 2-8 degree Celsius
SMILES: ClC=1C2=C(N=C(N1)NC(C(C)(C)C)=O)NC=C2
For R&D use only. Not for human or animal use.
N-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl)pivalamide; CAS No.: 149765-15-1; N-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl)pivalamide. PROPERTIES: N-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl)pivalamide appears as a white crystalline powder. Its molecular formula is C11H11ClN4O, corresponding to a molecular weight of 252.68 g/mol. The compound demonstrates moderate solubility in polar solvents such as water, methanol, and dimethylformamide, but limited solubility in non-polar solvents. It has a melting point typically ranging from 195-200 C and is stable under normal laboratory conditions when stored properly. Recommended storage involves keeping the material in a tightly sealed container at room temperature (15-25 C), preferably in a cool, dry, and well-ventilated area away from direct sunlight and moisture. The compound is hygroscopic to some extent and should be protected from excessive humidity. Safety precautions include wearing appropriate personal protective equipment such as chemical-resistant gloves and eye protection when handling the material. In case of skin contact, washing with soap and water is advised, and eye contact requires thorough rinsing with water for at least 15 minutes followed by medical attention if irritation persists. The compound should be handled in well-ventilated areas to avoid inhalation of dust particles. APPLICATIONS: N-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl)pivalamide serves as a valuable building block in pharmaceutical synthesis, particularly in the development of novel antiviral and anticancer agents. Its unique heterocyclic framework provides valuable bioisosteric properties that enhance binding affinity to various biological targets. In medicinal chemistry, the compound has been utilized as a core structure in the synthesis of viral polymerase inhibitors and as a scaffold for kinase inhibitors targeting oncogenic pathways, as reported in several pharmaceutical research journals. The pyrrolopyrimidine system offers versatile functionalization sites that facilitate the introduction of substituents to modulate solubility, metabolic stability, and target specificity. Additionally, N-(4-chloro-7H-pyrrolo[2,3-d]pyrimidin-2-yl)pivalamide finds application in materials science as a building block for organic semiconductors and in the development of fluorescent probes for bioimaging applications. Its utility extends to analytical chemistry, where it serves as a reference compound for spectral databases and as a starting material for custom syntheses in specialized laboratories focusing on heterocyclic chemistry and medicinal applications. The compound's structural features make it suitable for incorporation into molecular sensors and as a component in luminescent materials for display technologies.
Reviews
Write Your Own Review



![N-(4-chloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidin-2-yl)pivalamide](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196499_2.jpg)
![2-chloro-7H-pyrrolo[2,3-d]pyrimidine-6-carboxylic acid](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/2/0/201115_2.jpg)