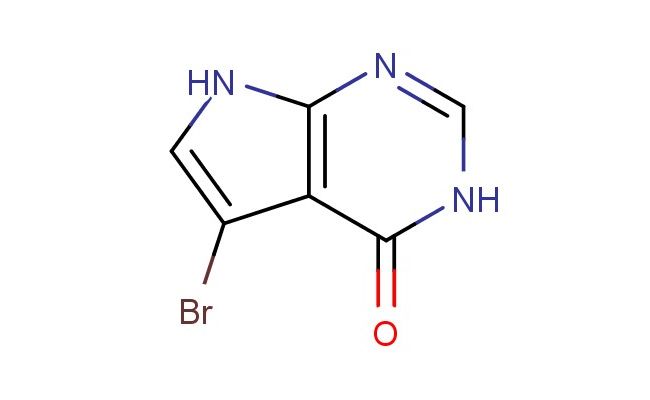
5-bromo-3H-pyrrolo[2,3-d]pyrimidin-4(7H)-one
$200.00
CAS No.: 22276-97-7
Catalog No.: 196496
Purity: 95%
MF: C6H4BrN3O
MW: 214.022
Storage: 2-8 degree Celsius
SMILES: BrC1=CNC=2N=CNC(C21)=O
Catalog No.: 196496
Purity: 95%
MF: C6H4BrN3O
MW: 214.022
Storage: 2-8 degree Celsius
SMILES: BrC1=CNC=2N=CNC(C21)=O
For R&D use only. Not for human or animal use.
5-bromo-3H-pyrrolo[2,3-d]pyrimidin-4(7H)-one; CAS No.: 22276-97-7; 5-bromo-3H-pyrrolo[2,3-d]pyrimidin-4(7H)-one. PROPERTIES: 5-bromo-3H-pyrrolo[2,3-d]pyrimidin-4(7H)-one appears as a white crystalline powder. Its molecular formula is C7H4BrN3O, corresponding to a molecular weight of 252.03 g/mol. The compound exhibits moderate solubility in common organic solvents such as dimethylformamide and dimethyl sulfoxide, but limited water solubility. It has a melting point typically ranging from 230-235 C and is stable under normal laboratory conditions when stored properly. Recommended storage involves keeping the material in a tightly sealed container at room temperature (15-25 C), preferably in a cool, dry, and well-ventilated area away from direct sunlight and moisture. The compound is light-sensitive to some extent and should be protected from prolonged exposure to UV light. Safety precautions include wearing appropriate personal protective equipment such as chemical-resistant gloves and eye protection when handling the material. In case of skin contact, washing with soap and water is advised, and eye contact requires thorough rinsing with water for at least 15 minutes followed by medical attention if necessary. The compound should be handled in well-ventilated areas to minimize inhalation risks. APPLICATIONS: 5-bromo-3H-pyrrolo[2,3-d]pyrimidin-4(7H)-one serves as a valuable building block in pharmaceutical chemistry, particularly in the development of targeted kinase inhibitors and nucleoside analogs. The bromo substituent provides a valuable site for cross-coupling reactions and bioisosteric replacements. In medicinal chemistry research, the compound has been utilized as a core structure in the synthesis of novel anticancer agents targeting specific oncogenic pathways, as documented in various pharmaceutical research publications. The pyrrolopyrimidinone framework offers a suitable platform for the design of bioactive molecules with improved binding affinity and selectivity. Additionally, 5-bromo-3H-pyrrolo[2,3-d]pyrimidin-4(7H)-one finds application in materials science as a building block for organic light-emitting diodes and in the development of fluorescent probes for bioimaging applications. Its utility extends to analytical chemistry, where it serves as a reference standard for chromatographic analyses and as a starting material for custom syntheses in specialized laboratories focusing on heterocyclic chemistry and medicinal applications. The compound's electronic properties make it suitable for incorporation into molecular sensors and as a component in luminescent materials for optical devices.
Reviews
Write Your Own Review



![4-chloro-7H-pyrrolo[2,3-d]pyrimidin-5-amine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196495_2.jpg)
![7-bromo-1H-pyrrolo[3,2-d]pyrimidin-4(5H)-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/196497_2.jpg)