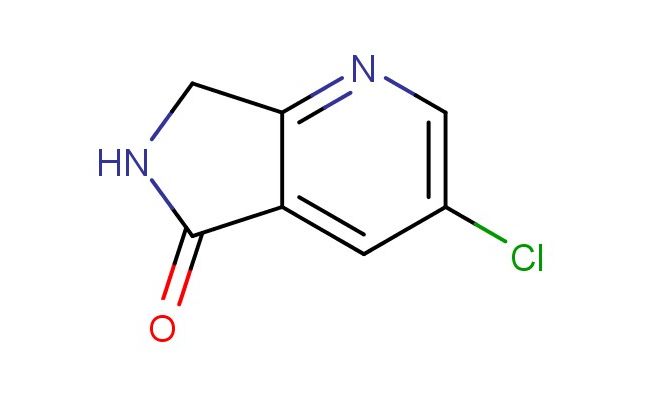
3-chloro-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one
$250.00
CAS No.: 1256806-34-4
Catalog No.: WLZ1219
Purity: 95%
MF: C7H5ClN2O
MW: 168.583
Storage: 2-8 degree Celsius
SMILES: O=C1NCC2=NC=C(Cl)C=C21
Catalog No.: WLZ1219
Purity: 95%
MF: C7H5ClN2O
MW: 168.583
Storage: 2-8 degree Celsius
SMILES: O=C1NCC2=NC=C(Cl)C=C21
For R&D use only. Not for human or animal use.
CAS NO.: 1256806-34-4; 3-chloro-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one. PROPERTIES: This chlorinated heterocycle features a chloro substituent on a fused pyrrole-pyridine system with partial hydrogenation, creating a molecule with potential pharmacological relevance. The 3-chloro-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one typically appears as a pale yellow crystalline solid with limited aqueous solubility but good solubility in polar aprotic solvents. Its molecular architecture includes a lactam functionality that imparts specific hydrogen bonding characteristics. For optimal stability and to preserve its structural integrity, this compound should be stored at 2-8 degree Celsius in a desiccator under anhydrous conditions. When handling, researchers should employ standard laboratory safety practices including nitrile gloves and a lab coat. This compound is hygroscopic and may degrade upon exposure to moisture. In case of skin contact, wash thoroughly with soap and water; if eye contact occurs, rinse immediately and seek medical advice. APPLICATIONS: The 3-chloro-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one serves as a lead compound in medicinal chemistry research targeting kinase inhibitors and other enzyme modulators. The chloro substituent provides a bioisosteric replacement that can enhance target binding affinity and selectivity. In pharmaceutical development, this compound functions as a starting material for synthesizing antiviral and anticancer agents where the fused heterocycle contributes to target engagement. Additionally, the molecule finds utility in chemical biology studies where its unique scaffold can be used to probe enzyme inhibition mechanisms and cellular signaling pathways. Researchers utilizing this compound benefit from its defined stereochemistry and functional group arrangement, advancing investigations into novel therapeutic approaches for various disease conditions.
Reviews
Write Your Own Review



![3,3-dibromo-1H-pyrrolo[2,3-b]pyridin-2(3H)-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/0/106640.jpg)
![4-chloro-1H-pyrrolo[2,3-b]pyridin-3-amine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/w/l/wlz2470_1.jpg)