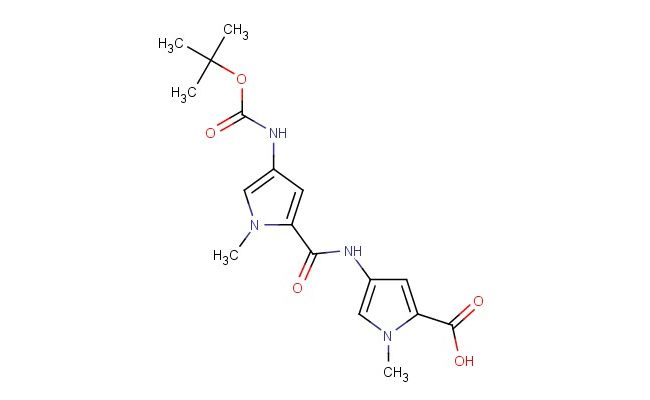
4-(4-((tert-butoxycarbonyl)amino)-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxylic acid
$300.00
CAS No.: 126092-98-6
Catalog No.: 196469
Purity: 95%
MF: C17H22N4O5
MW: 362.386
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)NC=1C=C(N(C1)C)C(=O)NC=1C=C(N(C1)C)C(=O)O
Catalog No.: 196469
Purity: 95%
MF: C17H22N4O5
MW: 362.386
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)NC=1C=C(N(C1)C)C(=O)NC=1C=C(N(C1)C)C(=O)O
For R&D use only. Not for human or animal use.
4-(4-((tert-butoxycarbonyl)amino)-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxylic acid; CAS No.: 126092-98-6; 4-(4-((tert-butoxycarbonyl)amino)-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxylic acid. PROPERTIES: 4-(4-((tert-butoxycarbonyl)amino)-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxylic acid features molecular formula C19H25N5O6 with molecular weight approximately 439.43 g/mol. It generally appears as a white to off-white powder with a reported melting point above 250 C. The compound is moderately hygroscopic and should be stored in a tightly sealed container at room temperature with a desiccant. Safety precautions include using chemical-resistant gloves and eye protection due to potential skin and eye irritation. The Boc-protected amine group requires acidic conditions for deprotection. In case of accidental ingestion, immediate medical consultation is advised. APPLICATIONS: 4-(4-((tert-butoxycarbonyl)amino)-1-methyl-1H-pyrrole-2-carboxamido)-1-methyl-1H-pyrrole-2-carboxylic acid functions as a specialized intermediate in peptide synthesis. Its Boc protection allows for sequential deprotection and coupling reactions. In medicinal chemistry, it has been employed in developing novel peptide-based anticancer agents, as reported in pharmaceutical research journals focusing on peptide drug development. The pyrrole carboxylic acid groups enable formation of amide bonds for peptide elongation. Additionally, this compound serves as a building block for chemical libraries used in high-throughput screening, with applications described in combinatorial chemistry literature focusing on diversity-oriented synthesis.
Reviews
Write Your Own Review