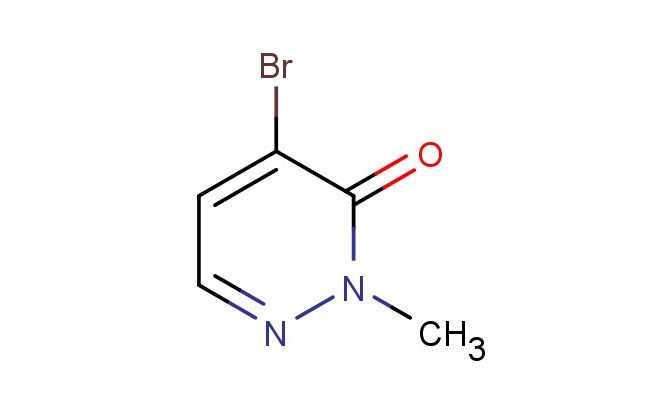
4-bromo-2-methylpyridazin-3(2H)-one
$325.00
CAS No.: 81816-98-0
Catalog No.: TQP2202
Purity: 95%
MF: C5H5BrN2O
MW: 189.012
Storage: 2-8 degree Celsius
SMILES: BrC=1C(N(N=CC1)C)=O
Catalog No.: TQP2202
Purity: 95%
MF: C5H5BrN2O
MW: 189.012
Storage: 2-8 degree Celsius
SMILES: BrC=1C(N(N=CC1)C)=O
For R&D use only. Not for human or animal use.
CAS NO.: 81816-98-0; 4-bromo-2-methylpyridazin-3(2H)-one. PROPERTIES: 4-bromo-2-methylpyridazin-3(2H)-one appears as pale yellow crystalline solids with characteristic pyridazine ring aroma. The compound features a molecular formula of C5H4BrN2O and a molecular weight of 205.01 g/mol. It demonstrates poor solubility in water but dissolves in DMSO and DMF with moderate ease. Storage at 2-8 degree Celsius in airtight containers is recommended to preserve its integrity, as the compound is somewhat hygroscopic and light-sensitive. When handling, protective eyewear and gloves are advisable due to its potential to cause mild skin and eye irritation. The substance is stable under nitrogen atmosphere but undergoes gradual decomposition when exposed to moist air. It has a flash point above 100 C, classifying it as a combustible solid. APPLICATIONS: 4-bromo-2-methylpyridazin-3(2H)-one functions as a versatile building block in heterocyclic chemistry. Its brominated position allows for cross-coupling reactions, enabling the synthesis of substituted pyridazine derivatives with diverse substituents. The methyl group provides steric and electronic modulation, influencing the reactivity of the pyridazine ring in subsequent transformations. In medicinal chemistry, this compound serves as a core structure for developing kinase inhibitors and other signaling pathway modulators, where the pyridazine scaffold contributes to hydrogen bonding interactions with target proteins. The bromine substituent facilitates Suzuki-Miyaura couplings, allowing for the introduction of aryl and heteroaryl groups to enhance pharmacokinetic properties. Additionally, the compound is employed in agrochemical research (though not for agricultural applications) as a starting material for developing herbicidal agents with novel modes of action. Its thermal stability makes it suitable for high-temperature coupling reactions without significant decomposition, preserving the integrity of the pyridazine system throughout synthetic sequences.
Reviews
Write Your Own Review