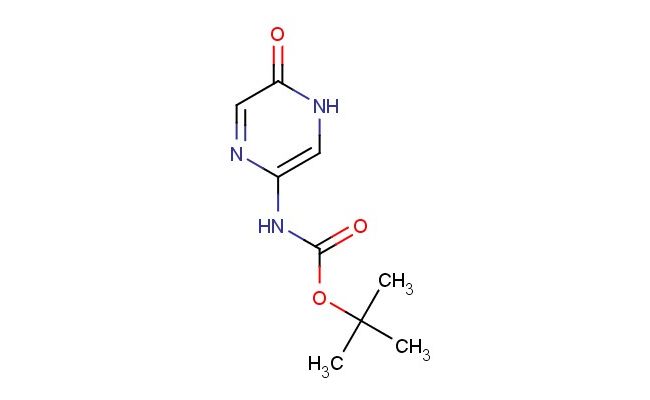
tert-Butyl (5-oxo-4,5-dihydropyrazin-2-yl)carbamate
$225.00
CAS No.: 2733641-59-1
Catalog No.: 192863
Purity: 95%
MF: C9H13N3O3
MW: 211.221
Storage: 2-8 degree Celsius
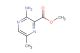
SMILES: O=C1NC=C(N=C1)NC(OC(C)(C)C)=O
Catalog No.: 192863
Purity: 95%
MF: C9H13N3O3
MW: 211.221
Storage: 2-8 degree Celsius
SMILES: O=C1NC=C(N=C1)NC(OC(C)(C)C)=O
For R&D use only. Not for human or animal use.
tert-Butyl (5-oxo-4,5-dihydropyrazin-2-yl)carbamate; CAS No.: 2733641-59-1; tert-Butyl (5-oxo-4,5-dihydropyrazin-2-yl)carbamate. PROPERTIES: This compound presents as a crystalline solid with molecular formula C9H14N2O3 and a molecular weight of 202.22 g/mol. It typically exhibits a melting point between 152-156 C and demonstrates moderate solubility in polar aprotic solvents such as dimethylformamide and dimethyl sulfoxide. The substance is sensitive to acidic hydrolysis and should be stored in neutral conditions. Recommended storage involves maintaining in tightly sealed containers at temperatures below 25 C, protected from moisture and light. When handling, standard laboratory safety precautions should be observed, including the use of gloves and eye protection, as tert-Butyl (5-oxo-4,5-dihydropyrazin-2-yl)carbamate may release irritating vapors upon heating. The carbamate group requires careful handling in strongly basic environments. APPLICATIONS: In pharmaceutical research, tert-Butyl (5-oxo-4,5-dihydropyrazin-2-yl)carbamate serves as a protected amine intermediate for synthesizing pyrazinone derivatives with potential CNS activity, as described in medicinal chemistry literature. Its oxo group enables participation in Michael addition reactions, making it valuable for constructing complex heterocyclic frameworks. Additionally, this compound functions as a key building block for preparing prodrugs designed to enhance bioavailability of pyrazine-based therapeutics. Academic studies have explored its utility in the development of kinase inhibitors, as reported in biochemical journals focusing on enzyme inhibition. The tert-butyl protecting group also facilitates controlled deprotection strategies in multi-step organic syntheses, as evidenced by methodological publications in synthetic chemistry.
Reviews
Write Your Own Review