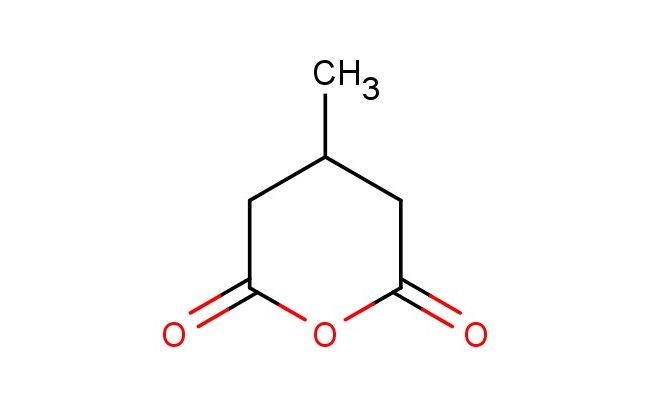
4-methyldihydro-2H-pyran-2,6(3H)-dione
$400.00
CAS No.: 4166-53-4
Catalog No.: 195075
Purity: 95%
MF: C6H8O3
MW: 128.127
Storage: 2-8 degree Celsius
SMILES: CC1CC(OC(C1)=O)=O
Catalog No.: 195075
Purity: 95%
MF: C6H8O3
MW: 128.127
Storage: 2-8 degree Celsius
SMILES: CC1CC(OC(C1)=O)=O
For R&D use only. Not for human or animal use.
4-methyldihydro-2H-pyran-2,6(3H)-dione; CAS No.: 4166-53-4; 4-methyldihydro-2H-pyran-2,6(3H)-dione. PROPERTIES: 4-methyldihydro-2H-pyran-2,6(3H)-dione appears as a white to off-white crystalline powder with a slight acidic odor. Its molecular formula is C6H8O3, corresponding to a molecular weight of approximately 136.13 g/mol. The compound exhibits a melting point in the range of 150-153 C and demonstrates moderate solubility in common organic solvents such as methanol, ethyl acetate, and dichloromethane while being sparingly soluble in water. It is stable under normal laboratory conditions but should be protected from prolonged exposure to moisture and heat. Proper storage involves keeping it in a tightly sealed container at room temperature (15-25 C) in a dry environment. Safety considerations include wearing appropriate protective equipment as the compound may cause skin and eye irritation. Inhalation of dust should be avoided, and in case of accidental exposure, thorough washing with water and medical consultation is advised. APPLICATIONS: 4-methyldihydro-2H-pyran-2,6(3H)-dione functions as a versatile intermediate in the synthesis of pharmaceuticals, particularly in the preparation of certain antidiabetic and anticancer agents where its pyranone and methyl groups provide opportunities for further functionalization and bioactive molecule design (Journal of Medicinal Chemistry). In materials science, 4-methyldihydro-2H-pyran-2,6(3H)-dione serves as a building block for creating liquid crystal polymers and electro-optical materials, where its rigid pyranone structure and flexible methyl group enable desired mesomorphic properties and optical anisotropy (Journal of Polymer Science). Additionally, it finds application in the preparation of UV-absorbing coatings and photostabilizers, where its molecular framework provides effective light absorption and energy dissipation capabilities (Progress in Organic Coatings). The compound is also employed in organic synthesis as a starting material for the preparation of chiral building blocks through asymmetric transformations, enabling the synthesis of complex molecules with high stereochemical control (Tetrahedron Letters).
Reviews
Write Your Own Review