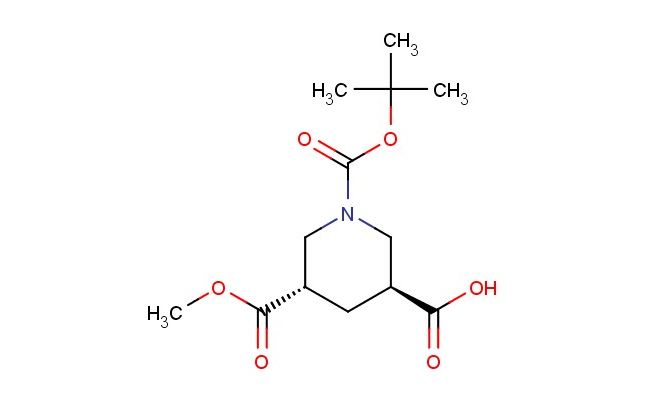
trans-1-[(tert-butoxy)carbonyl]-5-(methoxycarbonyl)piperidine-3-carboxylic acid
$400.00
CAS No.: 914261-00-0
Catalog No.: 200472
Purity: 95%
MF: C13H21NO6
MW: 287.312
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@H](C[C@@H](C1)C(=O)OC)C(=O)O
Catalog No.: 200472
Purity: 95%
MF: C13H21NO6
MW: 287.312
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@H](C[C@@H](C1)C(=O)OC)C(=O)O
For R&D use only. Not for human or animal use.
CAS NO.: 914261-00-0;trans-1-[(tert-butoxy)carbonyl]-5-(methoxycarbonyl)piperidine-3-carboxylic acid. PROPERTIES: This multifunctional compound appears as a white crystalline solid with a molecular weight of approximately 327.3 g/mol. The trans-1-[(tert-butoxy)carbonyl]-5-(methoxycarbonyl)piperidine-3-carboxylic acid features a piperidine ring substituted with Boc protection, methoxycarbonyl, and carboxylic acid groups in a trans configuration. It exhibits limited solubility in water but dissolves well in DMSO and DMF. Stability testing indicates sensitivity to acidic conditions and elevated temperatures, necessitating storage at 2-8 degree Celsius in a dry environment. When handling, personnel should use powder hoods, wear protective eyewear, and employ deliquescence-resistant tools. Skin contact may cause mild irritation, requiring thorough washing. Inhalation may lead to respiratory alkalosis, necessitating fresh air and medical evaluation. Eye exposure requires extended rinsing and possible corticosteroid treatment. Waste should be neutralized with sodium bicarbonate prior to disposal. APPLICATIONS: The trans-1-[(tert-butoxy)carbonyl]-5-(methoxycarbonyl)piperidine-3-carboxylic acid functions as a key intermediate in the synthesis of complex heterocycles for medicinal chemistry applications. Its Boc-protected amine and methoxycarbonyl groups provide versatile handles for further functionalization. The compound serves as a building block for creating macrocyclic peptides and enzyme inhibitors where precise spatial arrangement of functional groups is critical. Research institutions employ it as a precursor to bioactive molecules in combinatorial chemistry libraries. Additionally, its carboxylic acid group enables conjugation to biomolecules for creating targeted drug delivery systems. The trans configuration enhances its utility in creating rigidified structures with defined stereochemistry, making it valuable in the development of GPCR modulators and ion channel inhibitors.
Reviews
Write Your Own Review