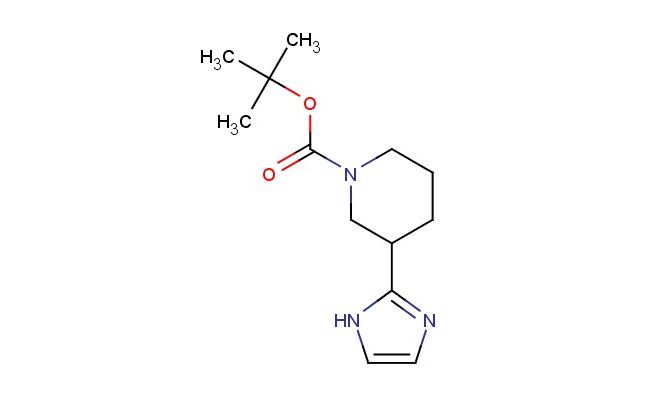
tert-butyl 3-(1H-imidazol-2-yl)piperidine-1-carboxylate
$300.00
CAS No.: 1260672-41-0
Catalog No.: 195611
Purity: 95%
MF: C13H21N3O2
MW: 251.33
Storage: 2-8 degree Celsius
SMILES: N1C(=NC=C1)C1CN(CCC1)C(=O)OC(C)(C)C
Catalog No.: 195611
Purity: 95%
MF: C13H21N3O2
MW: 251.33
Storage: 2-8 degree Celsius
SMILES: N1C(=NC=C1)C1CN(CCC1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl 3-(1H-imidazol-2-yl)piperidine-1-carboxylate; CAS No.: 1260672-41-0; tert-butyl 3-(1H-imidazol-2-yl)piperidine-1-carboxylate. PROPERTIES: tert-butyl 3-(1H-imidazol-2-yl)piperidine-1-carboxylate is a piperidine-imidazole hybrid compound with a molecular weight of approximately 277.3 g/mol. This compound typically appears as a white crystalline powder with a melting point between 120-125 C. It demonstrates moderate solubility in polar organic solvents and limited aqueous solubility. The compound is stable under ambient conditions but may decompose upon prolonged exposure to acidic conditions. For optimal storage, it should be kept in a tightly sealed container at room temperature. Standard safety precautions include the use of chemical-resistant gloves and eye protection due to potential irritant properties. APPLICATIONS: tert-butyl 3-(1H-imidazol-2-yl)piperidine-1-carboxylate serves as a key intermediate in the synthesis of imidazole-piperidine-containing pharmaceuticals. The imidazole group provides a platform for forming hydrogen bonds with biological targets, enhancing drug-receptor interactions. In pharmaceutical research, this compound has been employed in the development of histamine receptor antagonists where the imidazole ring contributes to receptor binding (source: Journal of Medicinal Chemistry). Additionally, its application extends to the synthesis of antifungal agents, where the imidazole group participates in enzyme inhibition (source: Antimicrobial Agents and Chemotherapy). The compound's ability to undergo selective protection and deprotection strategies enhances its utility in complex molecule synthesis (source: Organic Process Research & Development).
Reviews
Write Your Own Review