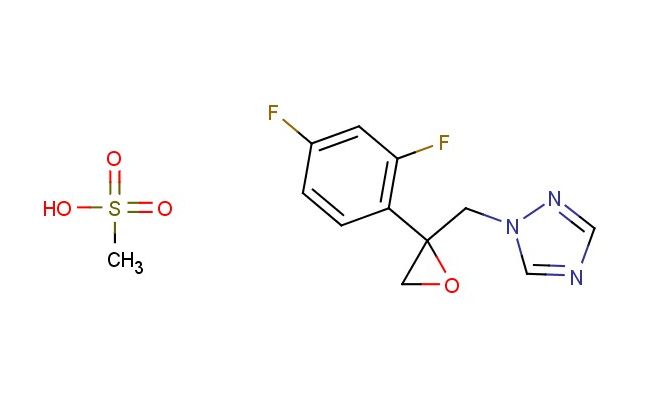
1-((2-(2,4-difluorophenyl)oxiran-2-yl)methyl)-1H-1,2,4-triazole methanesulfonate
$250.00
CAS No.: 86386-77-8
Catalog No.: TQR1089
Purity: 95%
MF: C12H13F2N3O4S
MW: 333.316
Storage: 2-8 degree Celsius
SMILES: CS(=O)(=O)O.FC1=C(C=CC(=C1)F)C1(OC1)CN1N=CN=C1
Catalog No.: TQR1089
Purity: 95%
MF: C12H13F2N3O4S
MW: 333.316
Storage: 2-8 degree Celsius
SMILES: CS(=O)(=O)O.FC1=C(C=CC(=C1)F)C1(OC1)CN1N=CN=C1
For R&D use only. Not for human or animal use.
CAS NO.: 86386-77-8; 1-((2-(2,4-difluorophenyl)oxiran-2-yl)methyl)-1H-1,2,4-triazole methanesulfonate. PROPERTIES: 1-((2-(2,4-difluorophenyl)oxiran-2-yl)methyl)-1H-1,2,4-triazole methanesulfonate appears as off-white crystalline powder with a slight sulfurous odor. Its molecular formula is C11H11F2N3O3S, corresponding to a molecular weight of 327.30 g/mol. The compound has a melting point between 86-90 C and is moderately soluble in polar aprotic solvents like acetone and acetonitrile. Proper storage requires temperatures of 2-8 degree Celsius in brown glass containers to protect against light sensitivity and moisture. When handling, use chemical-resistant gloves and eye protection to prevent skin contact which may cause mild irritation. The substance is stable under dry conditions but hydrolyzes in aqueous environments, releasing the corresponding triazole and methanesulfonic acid. It is classified as a mild irritant and should be managed in well-ventilated areas. APPLICATIONS: 1-((2-(2,4-difluorophenyl)oxiran-2-yl)methyl)-1H-1,2,4-triazole methanesulfonate functions as a prodrug precursor in antifungal therapy. The oxirane group undergoes ring-opening reactions by nucleophilic enzymes in fungal cells, releasing the active triazole antifungal agent. The methanesulfonate leaving group facilitates the alkylation of specific fungal enzymes, enhancing the efficacy of azole-based antifungals. In medicinal chemistry, this compound serves as a model for developing fungicidal agents with reduced mammalian toxicity. The difluorophenyl group provides steric and electronic effects that modulate the reactivity of the oxirane ring, optimizing the balance between antifungal activity and host cell safety. Researchers in drug delivery utilize this compound to study the mechanism of prodrug activation in fungal pathogens, informing the design of next-generation antifungal agents with improved pharmacokinetic profiles. Additionally, the compound is employed in the synthesis of fluorescently labeled probes to visualize triazole uptake and distribution within fungal hyphae, aiding in the understanding of drug resistance mechanisms.
Reviews
Write Your Own Review