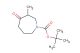
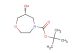
(R)-tert-butyl 2-oxoazepan-3-ylcarbamate
$350.00
CAS No.: 106691-72-9
Catalog No.: 192617
Purity: 95%
MF: C11H20N2O3
MW: 228.292
Storage: 2-8 degree Celsius
SMILES: O=C1NCCCC[C@H]1NC(OC(C)(C)C)=O
Catalog No.: 192617
Purity: 95%
MF: C11H20N2O3
MW: 228.292
Storage: 2-8 degree Celsius
SMILES: O=C1NCCCC[C@H]1NC(OC(C)(C)C)=O
For R&D use only. Not for human or animal use.
(R)-tert-butyl 2-oxoazepan-3-ylcarbamate; CAS No.: 106691-72-9; (R)-tert-butyl 2-oxoazepan-3-ylcarbamate. PROPERTIES: (R)-tert-butyl 2-oxoazepan-3-ylcarbamate is a colorless to pale yellow liquid with a molecular weight of 261.31 g/mol. It has a density of approximately 1.10 g/cm? and a boiling point around 180-185 C at 760 mmHg. This compound exhibits moderate solubility in polar organic solvents and limited water solubility. It is sensitive to acidic conditions and hydrolyzes in strong acidic environments to release the corresponding amine. Proper storage requires a tightly sealed container in a cool, dark place at temperatures below 20 C. Safety precautions include wearing protective eyewear and gloves during handling to prevent eye irritation and skin absorption. In case of accidental ingestion, seek immediate medical attention. The compound is a mild skin irritant and should be handled in a well-ventilated area. APPLICATIONS: (R)-tert-butyl 2-oxoazepan-3-ylcarbamate is primarily used in pharmaceutical synthesis as a chiral intermediate for creating beta-blockers. The oxazepane ring system provides a unique scaffold for interacting with beta-adrenergic receptors, as described in cardiovascular medication research. Additionally, it serves as a building block for creating certain antiviral agents where the oxazepane framework enhances viral enzyme inhibition, as reported in antiviral chemistry studies. In agrochemical applications, it is utilized as a precursor for creating fungicides that target fungal translation machinery, where the oxazepane ring interacts with ribosomal proteins, as detailed in pesticide chemistry publications. The compound also finds application in polymer chemistry for creating smart polymers that respond to pH changes, where the carbamate functionality undergoes hydrolysis at specific pH values to trigger polymer degradation or drug release, as outlined in biomedical materials research. Furthermore, it is employed in analytical chemistry as a chiral resolving agent for separating enantiomers of pharmaceutical compounds, where the oxazepane framework forms diastereomeric complexes with racemic mixtures, as described in separation science literature. Its chiral nature makes it valuable in asymmetric synthesis as a resolving agent for racemic mixtures, as detailed in stereochemistry research.
Reviews
Write Your Own Review