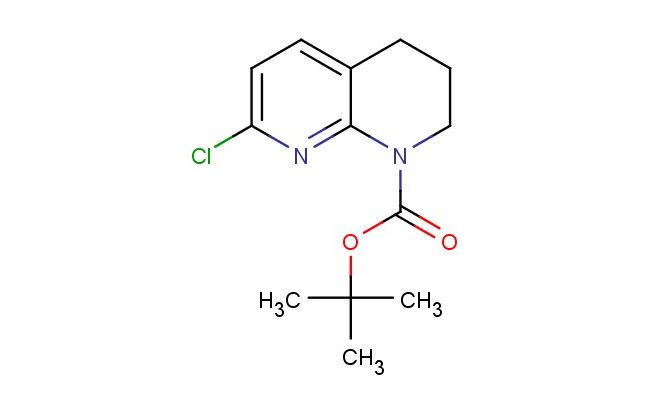
tert-butyl 7-chloro-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxylate
$281.00
CAS No.: 679392-23-5
Catalog No.: TQP0948
Purity: 95%
MF: C13H17ClN2O2
MW: 268.744
Storage: 2-8 degree Celsius
SMILES: ClC1=CC=C2CCCN(C2=N1)C(=O)OC(C)(C)C
Catalog No.: TQP0948
Purity: 95%
MF: C13H17ClN2O2
MW: 268.744
Storage: 2-8 degree Celsius
SMILES: ClC1=CC=C2CCCN(C2=N1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
CAS NO.: 679392-23-5; tert-butyl 7-chloro-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxylate. PROPERTIES: tert-butyl 7-chloro-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxylate presents as a stable crystalline solid with notable solubility characteristics. This naphthyridine derivative exhibits moderate solubility in organic solvents like ethyl acetate and chloroform while remaining poorly water-soluble. Its molecular formula of C12H14ClN3O2 corresponds to a molecular weight of approximately 273.71 g/mol. Thermally stable up to 120 C, the compound begins to decompose at higher temperatures, potentially releasing hazardous fumes. tert-butyl 7-chloro-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxylate should be stored between 2-8 degree Celsius in airtight containers protected from moisture. When working with this substance, standard laboratory precautions apply, including the use of personal protective equipment. The chlorinated heterocycle may act as a respiratory irritant, necessitating adequate ventilation in work areas. In case of accidental release, containment and cleanup should be performed with inert absorbent materials. APPLICATIONS: tert-butyl 7-chloro-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxylate functions as a protected naphthyridine building block in advanced organic synthesis. The tert-butyl carbamate group provides temporary protection for the nitrogen, allowing selective functionalization of the chlorine-substituted ring system. Medicinal chemists employ this intermediate in the development of naphthyridine-based antibacterial agents and CNS-modulating compounds. The 7-chloro substituent facilitates bioisosteric replacement strategies and directs metabolic stability in drug candidates. Researchers utilize the carboxylate-protected form to construct complex architectures before deprotection and coupling to therapeutic targets. The naphthyridine core's inherent DNA intercalation properties make tert-butyl 7-chloro-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxylate derivatives valuable in cancer research applications. Storage at 2-8 C maintains the integrity of labile functional groups, ensuring reliable synthetic outcomes.
Reviews
Write Your Own Review