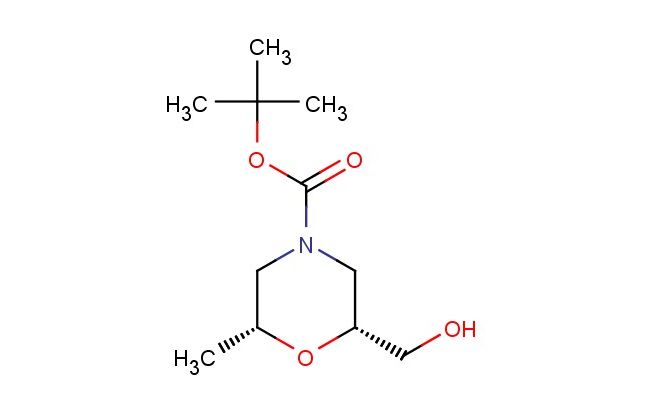
tert-butyl(2R,6R)-2-(hydroxymethyl)-6-methylmorpholine-4-carboxylate
$400.00
CAS No.: 1700609-48-8
Catalog No.: 200357
Purity: 95%
MF: C11H21NO4
MW: 231.292
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@@H](O[C@@H](C1)C)CO
Catalog No.: 200357
Purity: 95%
MF: C11H21NO4
MW: 231.292
Storage: 2-8 degree Celsius
SMILES: C(C)(C)(C)OC(=O)N1C[C@@H](O[C@@H](C1)C)CO
For R&D use only. Not for human or animal use.
CAS NO.: 1700609-48-8;tert-butyl(2R,6R)-2-(hydroxymethyl)-6-methylmorpholine-4-carboxylate. PROPERTIES: This enantiomerically pure compound appears as colorless crystals with moderate solubility in lower alcohols and ethyl acetate but poor water miscibility. The tert-butyl(2R,6R)-2-(hydroxymethyl)-6-methylmorpholine-4-carboxylate possesses a molecular weight around 257.3 g/mol, combining the morpholine heterocycle with a chiral quaternary center. Stability testing indicates vulnerability to basic hydrolysis and UV exposure, necessitating cold storage at 2-8 degree Celsius under nitrogen atmosphere in screw-capped vials. Safety protocols require lab coats, closed footwear, and face shields when handling bulk quantities. Accidental ingestion demands immediate medical attention with activated charcoal administration. Vapor accumulation should be prevented through adequate ventilation as inhalation may cause central nervous system depression. Waste disposal must comply with RCRA regulations for halogenated organics. APPLICATIONS: The tert-butyl(2R,6R)-2-(hydroxymethyl)-6-methylmorpholine-4-carboxylate functions as a versatile chiral synthon in advanced pharmaceutical synthesis, particularly in beta-blockers and CNS agents where its methyl-substituted morpholine provides enhanced metabolic stability. Its hydroxymethyl functionality enables glycosylation reactions for oligosaccharide assembly. Additionally, the compound serves as a masked diamine in the construction of macrolide antibiotics. The chiral backbone facilitates diastereoselective transformations in peptide mimetic design. Research teams leverage its reactivity profile for click chemistry conjugations in bioactive hybrid molecules. As a specialty chemical, it also finds application in the formulation of chiral catalysts for asymmetric epoxidation reactions, further amplifying its utility beyond traditional medicinal applications.
Reviews
Write Your Own Review