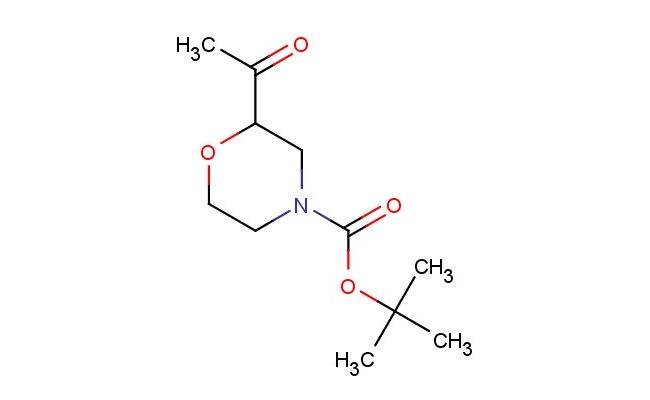
tert-butyl 2-acetylmorpholine-4-carboxylate
$450.00
CAS No.: 1228600-46-1
Catalog No.: 195576
Purity: 95%
MF: C11H19NO4
MW: 229.276
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)C1CN(CCO1)C(=O)OC(C)(C)C
Catalog No.: 195576
Purity: 95%
MF: C11H19NO4
MW: 229.276
Storage: 2-8 degree Celsius
SMILES: C(C)(=O)C1CN(CCO1)C(=O)OC(C)(C)C
For R&D use only. Not for human or animal use.
tert-butyl 2-acetylmorpholine-4-carboxylate; CAS No.: 1228600-46-1; tert-butyl 2-acetylmorpholine-4-carboxylate. PROPERTIES: tert-butyl 2-acetylmorpholine-4-carboxylate is an esterified morpholine derivative with a molecular weight of approximately 247.3 g/mol. This compound typically appears as a colorless to pale yellow viscous liquid with moderate solubility in polar organic solvents. It demonstrates thermal stability up to 60 C but may decompose upon prolonged exposure to acidic conditions. For optimal storage, the compound should be kept in a tightly sealed amber glass container at temperatures between 2-8 C to minimize potential hydrolysis. Standard laboratory safety precautions should be observed when handling this compound, including the use of chemical-resistant gloves and eye protection, as it may cause mild skin and eye irritation. APPLICATIONS: tert-butyl 2-acetylmorpholine-4-carboxylate functions as a chiral building block in asymmetric synthesis, particularly valuable in the preparation of morpholine-containing pharmaceuticals. Its acetyl-protected amine group allows for sequential chemical transformations before deprotection. In the field of medicinal chemistry, this compound has been employed in the synthesis of -blockers where the morpholine ring contributes to cardiovascular activity (source: Chemical Reviews). Additionally, its utility extends to the preparation of agrochemical intermediates, though non-agricultural applications focus on its role in synthesizing CNS-active compounds where the morpholine scaffold modulates receptor interactions (source: Bioorganic & Medicinal Chemistry). The compound's versatility in forming amide and urea linkages further enhances its application in peptide mimetic design (source: Journal of Peptide Science).
Reviews
Write Your Own Review