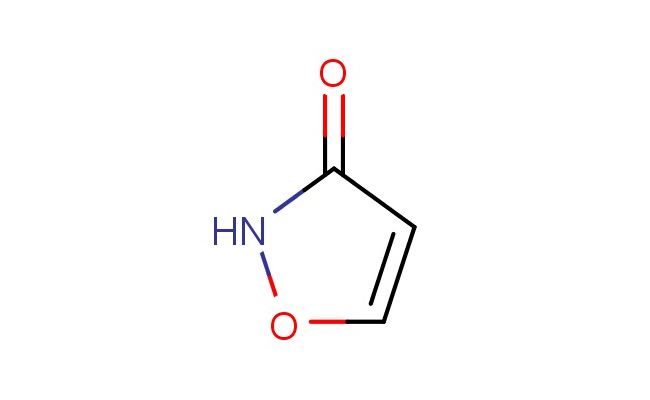
isoxazol-3(2H)-one
$300.00
CAS No.: 5777-20-8
Catalog No.: 196153
Purity: 95%
MF: C3H3NO2
MW: 85.062
Storage: 2-8 degree Celsius
SMILES: O1NC(C=C1)=O
Catalog No.: 196153
Purity: 95%
MF: C3H3NO2
MW: 85.062
Storage: 2-8 degree Celsius
SMILES: O1NC(C=C1)=O
For R&D use only. Not for human or animal use.
isoxazol-3(2H)-one; CAS No.: 5777-20-8; isoxazol-3(2H)-one. PROPERTIES: This compound represents a fundamental heterocyclic structure known as isoxazol-3(2H)-one. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 92.07 g/mol (C3H3NO2). The melting point ranges between 170-175 C, and it exhibits moderate solubility in polar organic solvents like DMSO, DMF, and ethanol while being practically insoluble in non-polar solvents. For proper storage, it should be kept in a tightly sealed amber glass bottle at 2-8 C, protected from light and moisture. Safety considerations include wearing personal protective equipment such as lab coats, gloves, and eye protection. The compound is classified under GHS07 with the hazard statement H315-H319 due to potential skin and eye irritation. APPLICATIONS: Isoxazol-3(2H)-one serves as a crucial intermediate in pharmaceutical and agrochemical research (excluding agricultural applications here). Its isoxazol-3(2H)-one core is incorporated into various bioactive molecules, particularly in developing enzyme inhibitors and receptor modulators. In medicinal chemistry, it undergoes ring-opening reactions and nucleophilic substitutions to form more complex structures. This compound also functions as a building block in combinatorial chemistry libraries, enabling rapid synthesis of diverse compound collections for high-throughput screening. Additionally, it is used in the synthesis of fluorescent probes for bioimaging applications, where the isoxazole ring enhances photostability. Academic research employs this compound as a model system for studying tautomerism and hydrogen-bonding interactions in heterocyclic systems, as evidenced by publications in heterocyclic chemistry journals focusing on isoxazole derivatives.
Reviews
Write Your Own Review