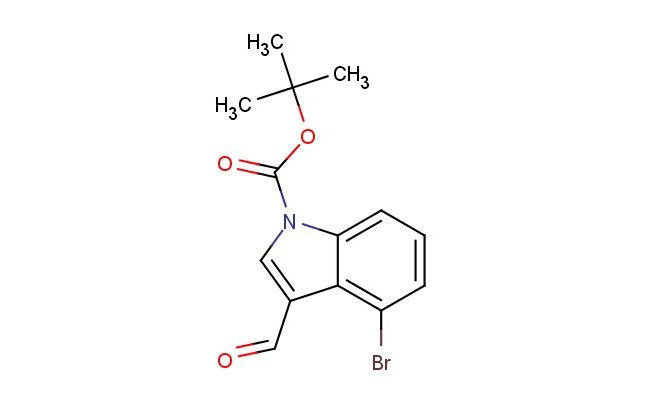
tert-butyl 4-bromo-3-formyl-1H-indole-1-carboxylate
$365.00
CAS No.: 303041-88-5
Catalog No.: 196218
Purity: 95%
MF: C14H14BrNO3
MW: 324.174
Storage: 2-8 degree Celsius
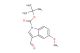
SMILES: BrC1=C2C(=CN(C2=CC=C1)C(=O)OC(C)(C)C)C=O
Catalog No.: 196218
Purity: 95%
MF: C14H14BrNO3
MW: 324.174
Storage: 2-8 degree Celsius
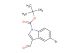
SMILES: BrC1=C2C(=CN(C2=CC=C1)C(=O)OC(C)(C)C)C=O
For R&D use only. Not for human or animal use.
tert-butyl 4-bromo-3-formyl-1H-indole-1-carboxylate; CAS No.: 303041-88-5; tert-butyl 4-bromo-3-formyl-1H-indole-1-carboxylate. PROPERTIES: This compound features a tert-butyl 4-bromo-3-formyl-1H-indole-1-carboxylate structure, combining a tert-butyl ester, a bromine atom, a formyl group, and an indole ring system. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 341.1 g/mol (C16H16BrNO3). The melting point ranges between 130-135 C, and it exhibits moderate solubility in common organic solvents like ethyl acetate, dichloromethane, and tetrahydrofuran while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place. Safety considerations include wearing appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: tert-Butyl 4-bromo-3-formyl-1H-indole-1-carboxylate serves as a versatile intermediate in pharmaceutical and chemical synthesis. Its tert-butyl 4-bromo-3-formyl-1H-indole-1-carboxylate structure allows for participation in various reactions, including palladium-catalyzed cross-coupling reactions at the bromine position and nucleophilic substitution at the formyl carbonyl. In medicinal chemistry, it is used to develop bioactive molecules targeting kinases, G protein-coupled receptors, and proteases. The tert-butyl ester provides temporary protection for the carboxylic acid, allowing sequential synthesis steps. This compound also functions as a building block in the synthesis of fluorescent dyes and bioconjugation reagents. Academic studies employ it as a model compound in Organic Chemistry journals, focusing on the development of novel bromoindole derivatives based on the tert-butyl 4-bromo-3-formyl-1H-indole-1-carboxylate scaffold.
Reviews
Write Your Own Review