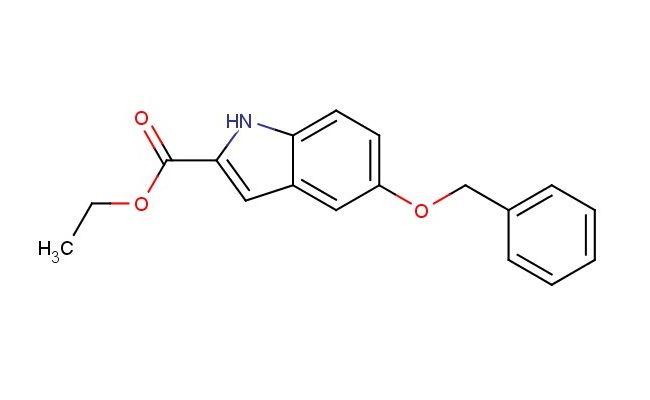
ethyl 5-benzyloxyindole-2-carboxylate
$250.00
CAS No.: 37033-95-7
Catalog No.: 195019
Purity: 95%
MF: C18H17NO3
MW: 295.338
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC=1C=C2C=C(NC2=CC1)C(=O)OCC
Catalog No.: 195019
Purity: 95%
MF: C18H17NO3
MW: 295.338
Storage: 2-8 degree Celsius
SMILES: C(C1=CC=CC=C1)OC=1C=C2C=C(NC2=CC1)C(=O)OCC
ethyl 5-benzyloxyindole-2-carboxylate; CAS No.: 37033-95-7; ethyl 5-benzyloxyindole-2-carboxylate. PROPERTIES: Ethyl 5-benzyloxyindole-2-carboxylate appears as a pale yellow to white crystalline solid with a slight characteristic odor. Its molecular formula is C19H17NO4, corresponding to a molecular weight of approximately 327.34 g/mol. The compound exhibits a melting point in the range of 100-103 C and demonstrates moderate solubility in common organic solvents such as methanol, ethyl acetate, and dichloromethane while being sparingly soluble in water. It is sensitive to acid and base hydrolysis, which may cleave the ester and benzyloxy groups. Proper storage requires keeping it in a tightly sealed container, preferably under an inert atmosphere like nitrogen, in a cool and dry environment at temperatures below 25 C. Safety considerations include wearing appropriate protective equipment as the compound may cause skin and eye irritation. Inhalation of dust should be avoided, and in case of accidental exposure, thorough washing with water and medical consultation is advised. APPLICATIONS: Ethyl 5-benzyloxyindole-2-carboxylate functions as a specialized intermediate in the synthesis of indole alkaloids and bioactive natural products, where its protected hydroxyl group and ester functionality enable sequential chemical transformations (Journal of Natural Products). In medicinal chemistry, ethyl 5-benzyloxyindole-2-carboxylate serves as a building block for creating serotonin receptor ligands and anti-inflammatory agents, leveraging its indole core which is a common pharmacophore in neuropsychiatric medications (Bioorganic & Medicinal Chemistry). Additionally, it finds application in the preparation of fluorescent indicators and sensors, where its indole moiety provides fluorescence response to environmental changes such as pH and ion concentration (Tetrahedron). The compound is also employed in materials science as a building block for creating organic semiconductors and field-effect transistors, where its aromatic structure and electron-withdrawing ester group enable charge transport properties (Journal of Materials Chemistry C).
Reviews
Write Your Own Review