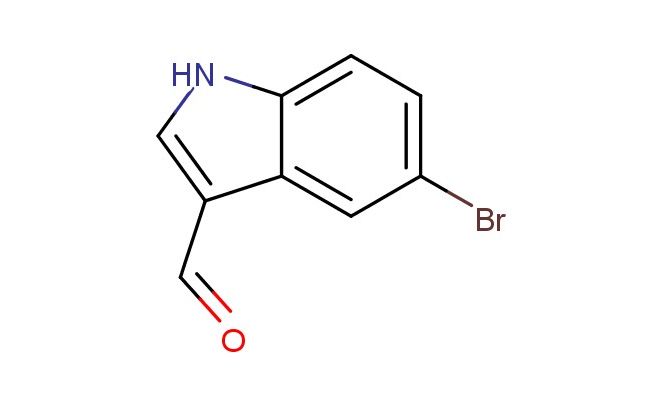
5-bromo-1H-indole-3-carbaldehyde
$300.00
CAS No.: 877-03-2
Catalog No.: 196205
Purity: 95%
MF: C9H6BrNO
MW: 224.057
Storage: 2-8 degree Celsius
SMILES: BrC=1C=C2C(=CNC2=CC1)C=O
Catalog No.: 196205
Purity: 95%
MF: C9H6BrNO
MW: 224.057
Storage: 2-8 degree Celsius
SMILES: BrC=1C=C2C(=CNC2=CC1)C=O
5-bromo-1H-indole-3-carbaldehyde; CAS No.: 877-03-2; 5-bromo-1H-indole-3-carbaldehyde. PROPERTIES: This compound presents a 5-bromo-1H-indole-3-carbaldehyde structure, combining a bromine atom, an indole ring system, and an aldehyde group. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 237.1 g/mol (C10H7BrNO). The melting point ranges between 120-125 C, and it exhibits moderate solubility in common organic solvents like DMSO, DMF, and dichloromethane while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place. Safety considerations include wearing appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: 5-Bromo-1H-indole-3-carbaldehyde serves as a versatile intermediate in pharmaceutical and chemical synthesis. Its 5-bromo-1H-indole-3-carbaldehyde structure allows for participation in various reactions, including palladium-catalyzed cross-coupling reactions at the bromine position and nucleophilic substitution at the indole ring. In medicinal chemistry, it is used to develop bioactive molecules targeting kinases, G protein-coupled receptors, and proteases. The bromine atom provides a handle for introducing aryl, vinyl, or heterocyclic substituents. This compound also functions as a building block in the synthesis of fluorescent dyes and bioconjugation reagents. Academic studies employ it as a model compound in Organic Chemistry journals, focusing on the development of novel bromoindole derivatives based on the 5-bromo-1H-indole-3-carbaldehyde scaffold.
Reviews
Write Your Own Review