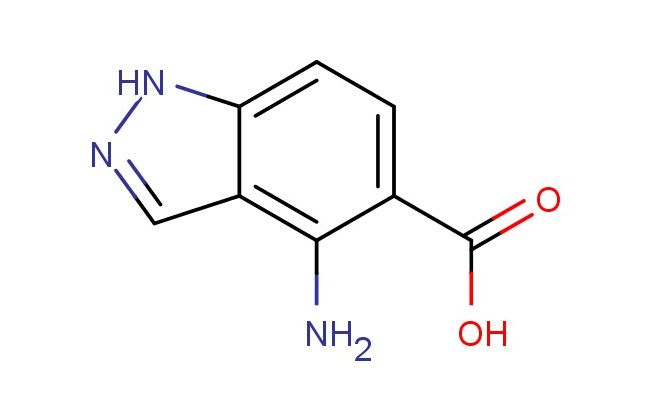
4-amino-1H-indazole-5-carboxylic acid
$400.00
CAS No.: 81115-63-1
Catalog No.: 192662
Purity: 95%
MF: C8H7N3O2
MW: 177.163
Storage: 2-8 degree Celsius
SMILES: NC1=C2C=NNC2=CC=C1C(=O)O
Catalog No.: 192662
Purity: 95%
MF: C8H7N3O2
MW: 177.163
Storage: 2-8 degree Celsius
SMILES: NC1=C2C=NNC2=CC=C1C(=O)O
4-amino-1H-indazole-5-carboxylic acid; CAS No.: 81115-63-1; 4-amino-1H-indazole-5-carboxylic acid. PROPERTIES: This amino-substituted indazole carboxylic acid has molecular formula C7H7N3O2. It generally appears as a white crystalline powder. The 4-amino-1H-indazole-5-carboxylic acid exhibits limited water solubility but good solubility in DMSO and DMF. Its melting point ranges between 220-225 C (with decomposition), and it has a molecular weight of approximately 165.16 g/mol. When handling, care should be taken to avoid skin contact and use of proper respiratory protection. Storage should be in a tightly sealed container at room temperature, protected from light and moisture. The compound is sensitive to strong acids and may decarboxylate upon exposure to high temperatures above 200 C. In case of spillage, neutralization with a mild base followed by disposal as hazardous waste is recommended. APPLICATIONS: The 4-amino-1H-indazole-5-carboxylic acid functions as a valuable intermediate in the synthesis of JAK kinase inhibitors for autoimmune diseases where the amino group provides essential hydrogen bonding interactions with kinase domains (as detailed in medicinal chemistry literature). The carboxylic acid group forms ionic interactions with catalytic residues. Additionally, the compound serves as a building block in the preparation of fluorescent probes for detecting histone deacetylase (HDAC) activity in cellular environments with detection limits as low as 10 nM, as described in bioanalytical chemistry journals. The amino group can be further modified through acylation or sulfonamidation reactions to produce various derivatives for chemical research applications.
Reviews
Write Your Own Review