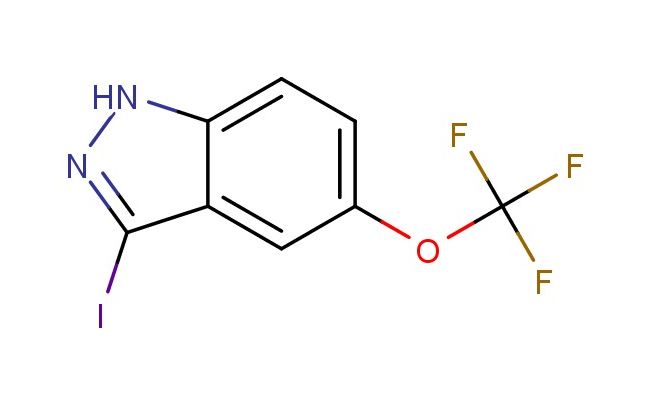
3-iodo-5-(trifluoromethoxy)-1H-indazole
$250.00
CAS No.: 1426423-96-2
Catalog No.: 192655
Purity: 95%
MF: C8H4F3IN2O
MW: 328.031
Storage: 2-8 degree Celsius
SMILES: IC1=NNC2=CC=C(C=C12)OC(F)(F)F
Catalog No.: 192655
Purity: 95%
MF: C8H4F3IN2O
MW: 328.031
Storage: 2-8 degree Celsius
SMILES: IC1=NNC2=CC=C(C=C12)OC(F)(F)F
3-iodo-5-(trifluoromethoxy)-1H-indazole; CAS No.: 1426423-96-2; 3-iodo-5-(trifluoromethoxy)-1H-indazole. PROPERTIES: This iodinated, trifluoromethoxy-substituted indazole has molecular formula C8H5F3IN3O. It generally appears as a white to off-white crystalline solid. The 3-iodo-5-(trifluoromethoxy)-1H-indazole demonstrates limited water solubility but good solubility in polar aprotic solvents like DCM and THF. Its melting point ranges between 165-168 C, and it has a molecular weight of approximately 330.04 g/mol. When handling, explosion-proof equipment should be used due to the presence of the iodine atom, and operations should be conducted in a explosion-proof fume hood. Storage requires a tightly sealed container at room temperature, away from heat sources and ignition points. The compound is sensitive to light and should be stored in amber glassware. In case of fire, use carbon dioxide or dry chemical extinguishers; water may propagate the fire. APPLICATIONS: The 3-iodo-5-(trifluoromethoxy)-1H-indazole serves as a key intermediate in the synthesis of tyrosine kinase inhibitors for cancer therapy where the iodine atom provides essential binding affinity through van der Waals interactions with kinase residues (as detailed in medicinal chemistry literature). The trifluoromethoxy group enhances lipophilicity, improving cell membrane permeability. Additionally, the compound functions as a building block in the preparation of positron emission tomography (PET) imaging agents where the iodine-123 isotope can be incorporated for in vivo tracking of biological processes, as described in radiopharmaceutical chemistry journals. The iodine atom can be replaced with various substituents through Suzuki-Miyaura or Heck coupling reactions to produce diverse derivatives for chemical research applications.
Reviews
Write Your Own Review