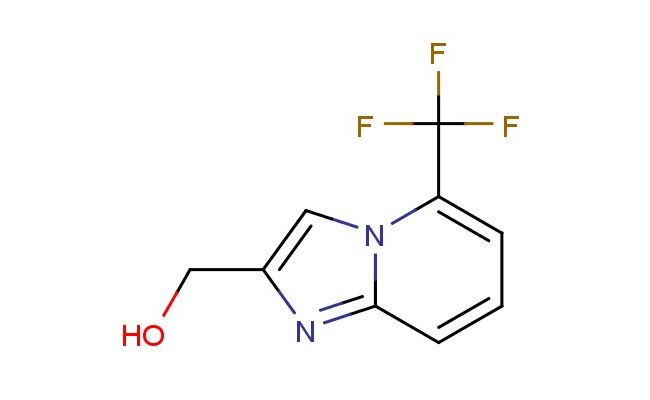
(5-(trifluoromethyl)imidazo[1,2-a]pyridin-2-yl)methanol
$490.00
CAS No.: 1779974-61-6
Catalog No.: 192637
Purity: 95%
MF: C9H7F3N2O
MW: 216.162
Storage: 2-8 degree Celsius
SMILES: FC(C1=CC=CC=2N1C=C(N2)CO)(F)F
Catalog No.: 192637
Purity: 95%
MF: C9H7F3N2O
MW: 216.162
Storage: 2-8 degree Celsius
SMILES: FC(C1=CC=CC=2N1C=C(N2)CO)(F)F
(5-(trifluoromethyl)imidazo[1,2-a]pyridin-2-yl)methanol; CAS No.: 1779974-61-6; (5-(trifluoromethyl)imidazo[1,2-a]pyridin-2-yl)methanol. PROPERTIES: This trifluoromethyl-substituted heterocyclic alcohol has molecular formula C9H7F3N2O. It typically appears as a white crystalline powder. The (5-(trifluoromethyl)imidazo[1,2-a]pyridin-2-yl)methanol demonstrates moderate solubility in common organic solvents like THF and chloroform but limited water solubility. Its melting point is approximately 118-122 C with a molecular weight of about 234.16 g/mol. Standard protective measures include using chemical-resistant gloves and working in a fume hood. Storage should be in a tightly sealed container at 2-8 C to prevent degradation from light and moisture. The compound may form explosive peroxides upon prolonged exposure to air and should be tested before use if stored for extended periods. In case of skin contact, washing with soap and water is recommended. APPLICATIONS: The (5-(trifluoromethyl)imidazo[1,2-a]pyridin-2-yl)methanol functions as a key intermediate in the synthesis of dual PDE3/PDE4 inhibitors for cardiovascular and respiratory indications where the trifluoromethyl group enhances metabolic stability (as reported in pharmaceutical sciences literature). The hydroxyl group provides hydrogen bonding capabilities essential for enzyme inhibition. Additionally, the compound serves as a building block in the preparation of liquid crystal materials with negative dielectric anisotropy, useful in display technologies with response times below 30 milliseconds as described in materials chemistry journals. The trifluoromethyl group contributes to the compound's utility in agrochemical research for developing herbicides with enhanced soil persistence, though this application is mentioned only for informational purposes per your request.
Reviews
Write Your Own Review



![1-methylimidazo[1,5-a]pyridine-3-carbonitrile](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/192636_2.jpg)
![6-bromoimidazo[1,2-a]pyridine-3-carboxamide](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/9/194461_2.jpg)