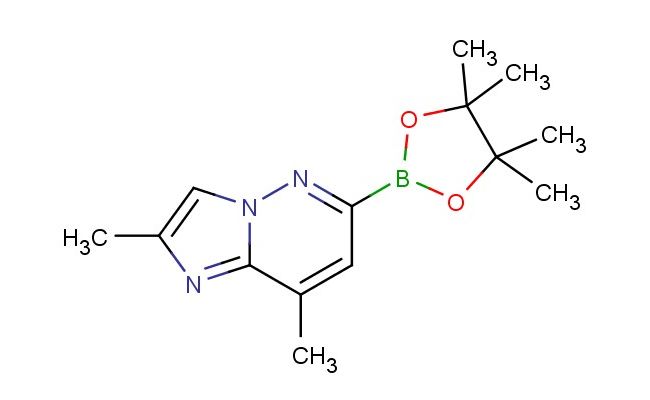
2,8-dimethyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)imidazo[1,2-b]pyridazine
$612.00
CAS No.: 1825352-86-0
Catalog No.: TQR0467
Purity: 95%
MF: C14H20BN3O2
MW: 273.145
Storage: 2-8 degree Celsius
SMILES: CC=1N=C2N(N=C(C=C2C)B2OC(C(O2)(C)C)(C)C)C1
Catalog No.: TQR0467
Purity: 95%
MF: C14H20BN3O2
MW: 273.145
Storage: 2-8 degree Celsius
SMILES: CC=1N=C2N(N=C(C=C2C)B2OC(C(O2)(C)C)(C)C)C1
For R&D use only. Not for human or animal use.
CAS NO.: 1825352-86-0; 2,8-dimethyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)imidazo[1,2-b]pyridazine. PROPERTIES: 2,8-dimethyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)imidazo[1,2-b]pyridazine appears as white crystalline powder with a slight ether-like odor. Its molecular formula is C15H19BN4O2, corresponding to a molecular weight of 304.14 g/mol. The compound has a melting point range of 115-119 C and is moderately soluble in THF and dioxane. Proper storage necessitates temperatures of 2-8 degree Celsius in airtight containers to prevent moisture-induced decomposition of the boronate ester group. When handling, use powder-free gloves and avoid inhalation as the compound may cause respiratory tract irritation. The substance is stable under inert atmosphere but reacts with water to release boronic acid and formaldehyde. It is classified as non-flammable but forms combustible dust clouds when dispersed in air. APPLICATIONS: 2,8-dimethyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)imidazo[1,2-b]pyridazine serves as a key intermediate in Suzuki-Miyaura coupling reactions. The boronate ester group provides a protected form of the boronic acid, which can be activated under mild conditions to form carbon-carbon bonds with aryl halides. In pharmaceutical research, this compound is used to develop kinase inhibitors where the imidazo[1,2-b]pyridazine scaffold facilitates hydrogen bonding interactions with the hinge region of kinase active sites. The dimethyl substitution enhances metabolic stability by reducing cytochrome P450 reactivity. In materials science, derivatives of this compound are employed in organic photovoltaics as electron-transport materials where the imidazopyridazine core contributes to efficient charge separation. Researchers in bioconjugation chemistry utilize this compound to create targeted therapeutic agents through bioorthogonal coupling reactions, enabling the attachment of drugs to biomolecules without affecting biological systems. Additionally, the compound functions as a building block for synthesizing fluorescent nucleic acid analogs used in gene expression studies, where the imidazopyridazine system provides photostable emission properties.
Reviews
Write Your Own Review



![6-chloroimidazo[1,2-b]pyridazin-2-ol](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/3/137068.jpg)
![6-bromo-2-methylimidazo[1,2-b]pyridazine](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/z/b/zb1825.jpg)