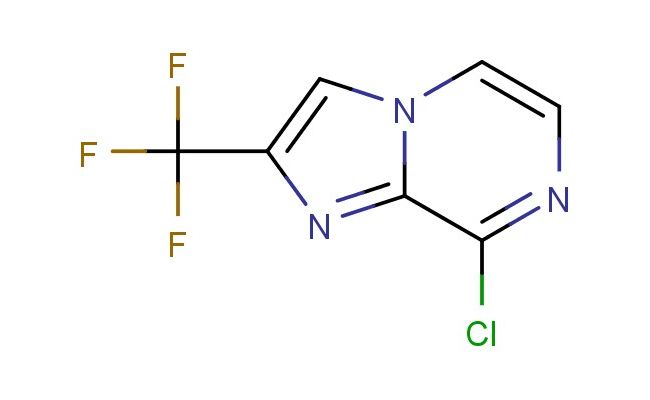
8-chloro-2-(trifluoromethyl)imidazo[1,2-a]pyrazine
$200.00
CAS No.: 611240-68-7
Catalog No.: HKP0001
Purity: 95%
MF: C7H3ClF3N3
MW: 221.569
Storage: 2-8 degree Celsius
SMILES: ClC=1C=2N(C=CN1)C=C(N2)C(F)(F)F
Catalog No.: HKP0001
Purity: 95%
MF: C7H3ClF3N3
MW: 221.569
Storage: 2-8 degree Celsius
SMILES: ClC=1C=2N(C=CN1)C=C(N2)C(F)(F)F
For R&D use only. Not for human or animal use.
CAS NO.: 611240-68-7;8-chloro-2-(trifluoromethyl)imidazo[1,2-a]pyrazine. PROPERTIES: This chlorinated heterocycle presents as a pale yellow solid with a molecular weight of approximately 226.6 g/mol. The 8-chloro-2-(trifluoromethyl)imidazo[1,2-a]pyrazine combines a chloro and trifluoromethyl substituents on a fused imidazopyrazine ring system. It exhibits limited aqueous solubility but good dissolution in polar aprotic solvents like THF and CHCl3. Stability characterization reveals sensitivity to nucleophilic aromatic substitution and base-catalyzed ring-opening, necessitating storage at 2-8 degree Celsius in amber glass containers. Handlers should employ powder hoods with HEPA filtration and use cut-resistant gloves during handling. Skin contact may cause mild irritation requiring thorough washing. Inhalation may induce respiratory tract irritation; treatment includes fresh air and medical evaluation. Eye exposure requires extended rinsing and possible corticosteroid application. Waste should be hydrolyzed with dilute acid prior to disposal. APPLICATIONS: The 8-chloro-2-(trifluoromethyl)imidazo[1,2-a]pyrazine functions as a key intermediate in the synthesis of various pharmaceuticals. Its fused heterocycle provides privileged structure characteristics for receptor tyrosine kinase targeting. Research teams utilize this compound as a starting material for creating kinase inhibitors and ion channel modulators. The chloro and trifluoromethyl groups enhance metabolic resistance and binding affinity in resulting drug candidates. Additionally, the compound undergoes Buchwald-Hartwig amination for constructing anilinic systems. Its unique substitution pattern makes it valuable in the development of fluorescent probes and bioimaging agents.
Reviews
Write Your Own Review



![(6-bromoimidazo[1,2-a]pyrazin-2-yl)methanol](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/g/s/gs3988_1.jpg)
![2-isopropylimidazo[1,2-a]pyrazin-8(7H)-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/h/k/hkp0002_1.jpg)