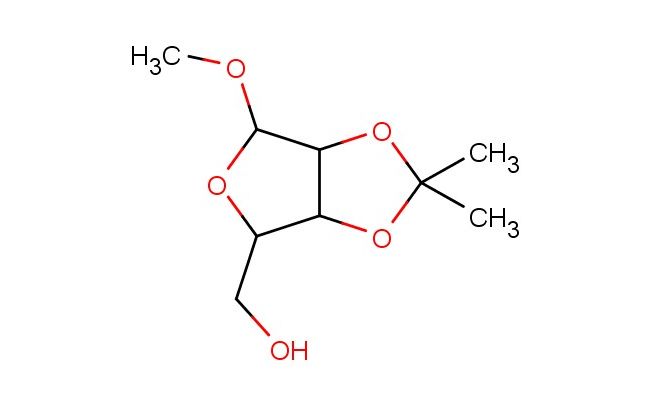
(6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol
$250.00
CAS No.: 4099-85-8
Catalog No.: 196177
Purity: 95%
MF: C9H16O5
MW: 204.222
Storage: 2-8 degree Celsius
SMILES: COC1OC(C2C1OC(O2)(C)C)CO
Catalog No.: 196177
Purity: 95%
MF: C9H16O5
MW: 204.222
Storage: 2-8 degree Celsius
SMILES: COC1OC(C2C1OC(O2)(C)C)CO
For R&D use only. Not for human or animal use.
(6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol; CAS No.: 4099-85-8; (6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol. PROPERTIES: This compound presents a (6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol structure, combining a methoxy group, dimethyl substituents, a tetrahydrofuro[3,4-d][1,3]dioxol ring system, and a hydroxymethyl group. It typically appears as a colorless to pale yellow viscous liquid with a molecular weight of approximately 222.2 g/mol (C9H16O5). The density is around 1.2 g/cm?, and it has a boiling point of approximately 220-225 C at 760 mmHg. It exhibits moderate solubility in common organic solvents like ethyl acetate, dichloromethane, and tetrahydrofuran while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place. Safety considerations include wearing appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: (6-Methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol serves as a specialized intermediate in pharmaceutical and chemical synthesis. Its (6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol structure provides a protected polyol framework suitable for developing complex carbohydrates and glycosides. In medicinal chemistry, it is used to create bioactive molecules with specific carbohydrate mimetic properties, particularly in projects targeting glycoprotein processing enzymes. The hydroxymethyl group can undergo etherification, esterification, or oxidation to carboxylic acid. This compound also functions as a building block in the synthesis of macrocyclic compounds and natural product-inspired architectures. Academic studies employ it as a model system in Organic Chemistry journals, focusing on the development of novel synthetic routes and reactivities of (6-methoxy-2,2-dimethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)methanol derivatives.
Reviews
Write Your Own Review



![(3aR,6R,6aR)-6-(hydroxymethyl)-2,2-dimethyldihydrofuro[3,4-d][1,3]dioxol-4(3aH)-one](https://www.chemshuttle.com/media/catalog/product/cache/31dbf0bffbfa69a5826a72cec9a446de/1/8/186105_2.jpg)