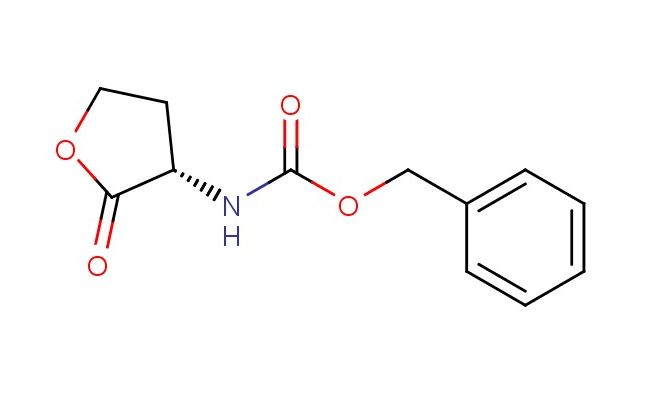
(S)-benzyl 2-oxotetrahydrofuran-3-ylcarbamate
$200.00
CAS No.: 35677-89-5
Catalog No.: 196180
Purity: 95%
MF: C12H13NO4
MW: 235.239
Storage: 2-8 degree Celsius
SMILES: O=C1OCC[C@@H]1NC(OCC1=CC=CC=C1)=O
Catalog No.: 196180
Purity: 95%
MF: C12H13NO4
MW: 235.239
Storage: 2-8 degree Celsius
SMILES: O=C1OCC[C@@H]1NC(OCC1=CC=CC=C1)=O
For R&D use only. Not for human or animal use.
(S)-benzyl 2-oxotetrahydrofuran-3-ylcarbamate; CAS No.: 35677-89-5; (S)-benzyl 2-oxotetrahydrofuran-3-ylcarbamate. PROPERTIES: This compound presents a (S)-benzyl 2-oxotetrahydrofuran-3-ylcarbamate structure, featuring a chiral center, a benzyl protecting group, a carbamate functionality, and an oxetane ring system. It typically appears as a white to off-white crystalline solid with a molecular weight of approximately 253.3 g/mol (C13H13NO4). The melting point ranges between 100-105 C, and it exhibits moderate solubility in common organic solvents like ethyl acetate, dichloromethane, and tetrahydrofuran while being sparingly soluble in water. Proper storage requires a tightly sealed container in a cool, dry place. Safety considerations include wearing appropriate PPE. It is classified as a skin and eye irritant (GHS07) with the hazard statement H315-H319. APPLICATIONS: (S)-Benzyl 2-oxotetrahydrofuran-3-ylcarbamate serves as a specialized intermediate in pharmaceutical and chemical synthesis. Its (S)-benzyl 2-oxotetrahydrofuran-3-ylcarbamate structure provides a chiral, protected amino alcohol framework suitable for developing enantioselective catalysts and bioactive molecules. In medicinal chemistry, it is used to create compounds targeting G protein-coupled receptors and ion channels. The carbamate group can undergo deprotection to release an amine or participate in urea bond formation. This compound also functions as a building block in the synthesis of complex carbohydrates and glycosides. Academic studies employ it as a model compound in Organic Chemistry journals, focusing on asymmetric synthesis and the development of novel chiral architectures based on the (S)-benzyl 2-oxotetrahydrofuran-3-ylcarbamate scaffold.
Reviews
Write Your Own Review